 |
 |
- Search
| J. People Plants Environ > Volume 26(6); 2023 > Article |
|
ABSTRACT
Background and objective: Apocynum lancifolium Russanov is a perennial herb native to Korea and belonging to the family Apocynaceae. Herein, cutting mediums and plant growth regulators (PGRs) were examined to investigate the requirements for the cutting propagation of A. lancifolium.
Methods: The study was conducted over a period of 6 weeks from May to June 2023. As types of cutting medium for propagation by cutting, Kanuma soil, commercial potting mixes, perlite, and peat moss were used, and the PGRs of indole-3-butyric acid (IBA) at varying concentrations (100, 500, and 1,000 mg┬ĘLŌłÆ1) and a 1:1 (v/v) solution of Rootone (0.4% 1-naphthylacetamide) diluted in water were applied for this study. After six weeks, growth characteristics, including cutting survival rate, rooting rate, shoot height, the number of leaves, root length, the number of roots, dry weight of shoots, and dry weight of roots, were examined.
Results: Among the different growing substrates used for experimentation, the cutting survival rate on Kanuma soil (81.7 ┬▒ 5.0%) was the highest, followed by commercial potting mixes (40.0 ┬▒ 7.2%) and perlite (66.7 ┬▒ 2.7%), while the lowest cutting survival rate was on peat moss (35.0 ┬▒ 6.3%). On treatment with Kanuma soil (81.7 ┬▒ 5.0%), the rooting rate was also significantly greater than that when treated with other substrates. In the PGR experiment, the cutting survival rate and rooting rate treated with Rootone (0.4% 1-naphthylacetamide) were significantly higher than those when treated with other substrates. Further, the number of roots increased with an increase in the IBA concentration. Based on the results, we recommend treating A. lancifolium cuttings with Rootone and Kanuma soil to ensure successful cutting propagation of A. lancifolium.
Conclusion: This mass production system for A. lancifolium via cutting propagation shows promise in terms of scalability and could help with germplasm conservation efforts for A. lancifolium.
Methods: The study was conducted over a period of 6 weeks from May to June 2023. As types of cutting medium for propagation by cutting, Kanuma soil, commercial potting mixes, perlite, and peat moss were used, and the PGRs of indole-3-butyric acid (IBA) at varying concentrations (100, 500, and 1,000 mg┬ĘLŌłÆ1) and a 1:1 (v/v) solution of Rootone (0.4% 1-naphthylacetamide) diluted in water were applied for this study. After six weeks, growth characteristics, including cutting survival rate, rooting rate, shoot height, the number of leaves, root length, the number of roots, dry weight of shoots, and dry weight of roots, were examined.
Results: Among the different growing substrates used for experimentation, the cutting survival rate on Kanuma soil (81.7 ┬▒ 5.0%) was the highest, followed by commercial potting mixes (40.0 ┬▒ 7.2%) and perlite (66.7 ┬▒ 2.7%), while the lowest cutting survival rate was on peat moss (35.0 ┬▒ 6.3%). On treatment with Kanuma soil (81.7 ┬▒ 5.0%), the rooting rate was also significantly greater than that when treated with other substrates. In the PGR experiment, the cutting survival rate and rooting rate treated with Rootone (0.4% 1-naphthylacetamide) were significantly higher than those when treated with other substrates. Further, the number of roots increased with an increase in the IBA concentration. Based on the results, we recommend treating A. lancifolium cuttings with Rootone and Kanuma soil to ensure successful cutting propagation of A. lancifolium.
Conclusion: This mass production system for A. lancifolium via cutting propagation shows promise in terms of scalability and could help with germplasm conservation efforts for A. lancifolium.
Apocynum lancifolium Russanov is a perennial herb and a subspecies of A. venetum belonging to the Apocynaceae family. Known as a halotolerant plant, A. lancifolium thrives in various environments, including coastal or reclaimed lands, deserts, and salt marshes (Son et al., 2011). In South Korea, it naturally grows along the western and southern coasts, and in the Gyeonggi-do region. Moreover, occasional inland distribution has been observed in Danyanggun, Chungcheongbuk-do (Kim et al., 2021a). A. lancifolium is renowned for its medicinal properties, which include cardioprotective, antihypertensive, hepatoprotective, and antidepressant functions. Ongoing research has focused on its potential as a natural cosmetic ingredient in the aesthetics industry (Walternberger et al., 2016; Fan et al., 1999; Zheng et al., 2012). The Korea Forest Service currently designates A. lancifolium as an endangered species, thus, underscoring the importance of conserving this native plant of South Korea.
According to the initial report of the National Garden Promotion Plan by the Korea Forest Service (2016), the horticulture industry has steadily expanded, both domestically and internationally. Recently, South Korea has seen increased interest in horticulture and garden construction. With the implementation of the Nagoya Protocol, there is a growing need to protect biosovereignty that can reduce the reliance on foreign bioresources and mitigate royalty payments for imported horticultural species. Oh et al. (2021) highlighted that out of 4,000 native plants in South Korea, approximately only 600 exhibit significant development potential, with few currently in use. To realize the full potential of native plants, there must be sufficient production capacity to meet the increasing demands, thereby necessitating the development of advanced propagation techniques for mass production.
Among various methods employed for plant reproduction, cutting propagation promotes mass propagation without introducing genetic mutations. Thus, this method can be readily employed at farms or seed nurseries to accelerate the development of new cultivars (Oh et al., 2021). Several factors influence the rooting rate and root development during cutting propagation, including plant growth regulators (PGRs), timing of cuttings, propagation media, and humidity (Kim and Kim, 2012). While Kim et al. (2021) examined the effects of NaCl on seed germination in A. lancifolium during propagation, the cutting propagation method for this species has not yet been investigated. Research has shown that the seed germination rate of A. lancifolium is generally high; however, its mass production is challenging due to the relatively low survival rate of germinated seedlings. Additionally, as germination rates decline with reduced vitality during seed storage, limitations exist in terms of reproduction. Therefore, the status of seed germination for A. lancifolium suggests the need for more systematic and efficient research. Accordingly, this study aimed to investigate the impact of propagation media and PGRs (rooting agents) on the rooting of A. lancifolium, to establish a system for the vegetative reproduction of this native plant species.
To prepare scion samples, softwood from 2-year-old A. lancifolium plants was collected from the Sejong National Arboretum greenhouse in May 2023. To prevent water loss, the collected softwood was soaked in water for subsequent experiments, and to minimize transpiration, one of the two leaves on each scion was removed (Fig. 1). Scion lengths were standardized to 6ŌĆō7 cm (2ŌĆō3 nodes), and the base was cut diagonally. Scions were then buried in a propagation box (52 cm ├Ś 36.5 cm ├Ś 9 cm) to a depth of approximately 3 cm. The cutting propagation experiments were conducted in a solar-heated greenhouse equipped with an automatic fog sprayer to maintain constant air humidity. All scions were drip-irrigated once per day and the amount of water was approximately 1,000 ml per propagation box. A shading net is installed in the greenhouse to avoid direct sunlight falling on scions and maintain an average natural light intensity of approximately 115 ╬╝mol/s/m2. Mean relative humidity and temperature in the greenhouse were maintained at 75 ┬▒ 5% and 25 ┬▒ 5┬░C, respectively.
To assess the impact of propagation media on A. lancifolium rooting, the central portion of the scion was used, excluding the apical and basal regions of the stem. The propagation media included Kanuma soil as volcanic ash soil, commercial potting mixes, perlite, and peat moss (Table 1). Each medium was filled in a propagation box, and the cutting propagation was replicated four times, with 15 samples per treatment. The boxes were placed on their respective plates, and after six weeks, growth characteristics, including cutting survival rate, rooting rate, shoot height, the number of leaves, root length, the number of roots, dry weight of shoots, and dry weight of roots, were examined.
The middle section of the scion, excluding the apical and basal areas of the stem, was used to investigate the effects of PGRs on A. lancifolium rooting. The basal part of the scion was immersed in IBA (indole-3-butyric acid, Duchefa, Haarlem, The Netherlands) at varying concentrations (100, 500, and 1,000 mg┬ĘLŌłÆ1) for 30 min. Additionally, a 1:1 (v/v) solution of Rootone (0.4% 1-naphthylacetamide, Biosciencekorea, Seoul, Korea) diluted in water was applied for the same 30-min immersion period. Cutting propagation was conducted across five treatments, including a control treatment using Kanuma soil, with four replicates and 15 samples per treatment. The propagation boxes were positioned on their respective plates, and after six weeks, growth characteristics were evaluated. Experimental conditions and growth parameters were consistent with those of the propagation media experiment.
Data from the experiments were analyzed using the R program (version 4.2.1). Analysis of variance was performed, and TukeyŌĆÖs honestly significant difference test was applied to assess the statistical significance of mean values among different treatments for comparison. Percentage data were arcsine-transformed for analysis (Anderson and McLean, 1974).
The results of the analysis on the effects of propagation media on A. lancifolium scions are presented in Fig. 2 and Table 2. The cutting survival rate of A. lancifolium scions was the highest when Kanuma soil was used as the propagation medium, followed by perlite, commercial potting mixes, and peat moss (81.7 ┬▒ 5.0%, 66.7 ┬▒ 2.7%, 40.0 ┬▒ 7.2%, and 35.0 ┬▒ 6.3%, respectively). Kanuma soil also exhibited a significantly high rooting rate and root length (81.7 ┬▒ 5.0% and 36.2 ┬▒ 2.5 mm, respectively). Contrastingly, peat moss showed a 0% rooting rate. The highest shoot height (69.1 ┬▒ 14.3) was observed for commercial potting mixes, followed by Kanuma soil (41.1 ┬▒ 5.1), perlite (29.9 ┬▒ 2.0), and peat moss (14.4 ┬▒ 1.8). Water-holding capacity and permeability should be properly balanced in the soil during the plant growth period. The propagation media has been well known to affect anchoring the cutting in place, holding water for the cutting, supplying sufficient aeration for adventitious rooting (Hartmann et al. 2011). According to Erstad and Gislerod (1994), water in excess prevents proper aeration. Given our results and previous studies, we speculate that peat moss contains high water-holding capacity, which had a negative effect on the rooting of A. lancifolium. The most suitable conditions for plant growth are 20ŌĆō30% air volume with a porosity of 85% or more (Gruda and Schnitzler, 2004). When mixing peat moss and perlite, the air porosity was higher than 100% peat moss when mixing peat moss and perlite (Verdonck and Demeyer, 2004). In addition, the importance of changes in physical properties depending on the components of the media is recognized and research on this has been conducted consistently. Lee et al. (2009) reported that water-holding capacity was the most critical factor for the rooting of Hydrangea serrata for. acuminata, while Lee and Lee (2007) found that a high proportion of peat moss was advantageous for the rooting of highbush blueberries. In this study, although Kanuma soil showed a positive effect on the root induction and development, a combination of commercial potting mixes and Kanuma soil in an appropriate ratio is recommended for considering overall plant growth of A. lancifolium. The ideal mixture of media varies according to the plant species; nonetheless, further research is needed to explore the effects of physicochemical properties of propagation media.
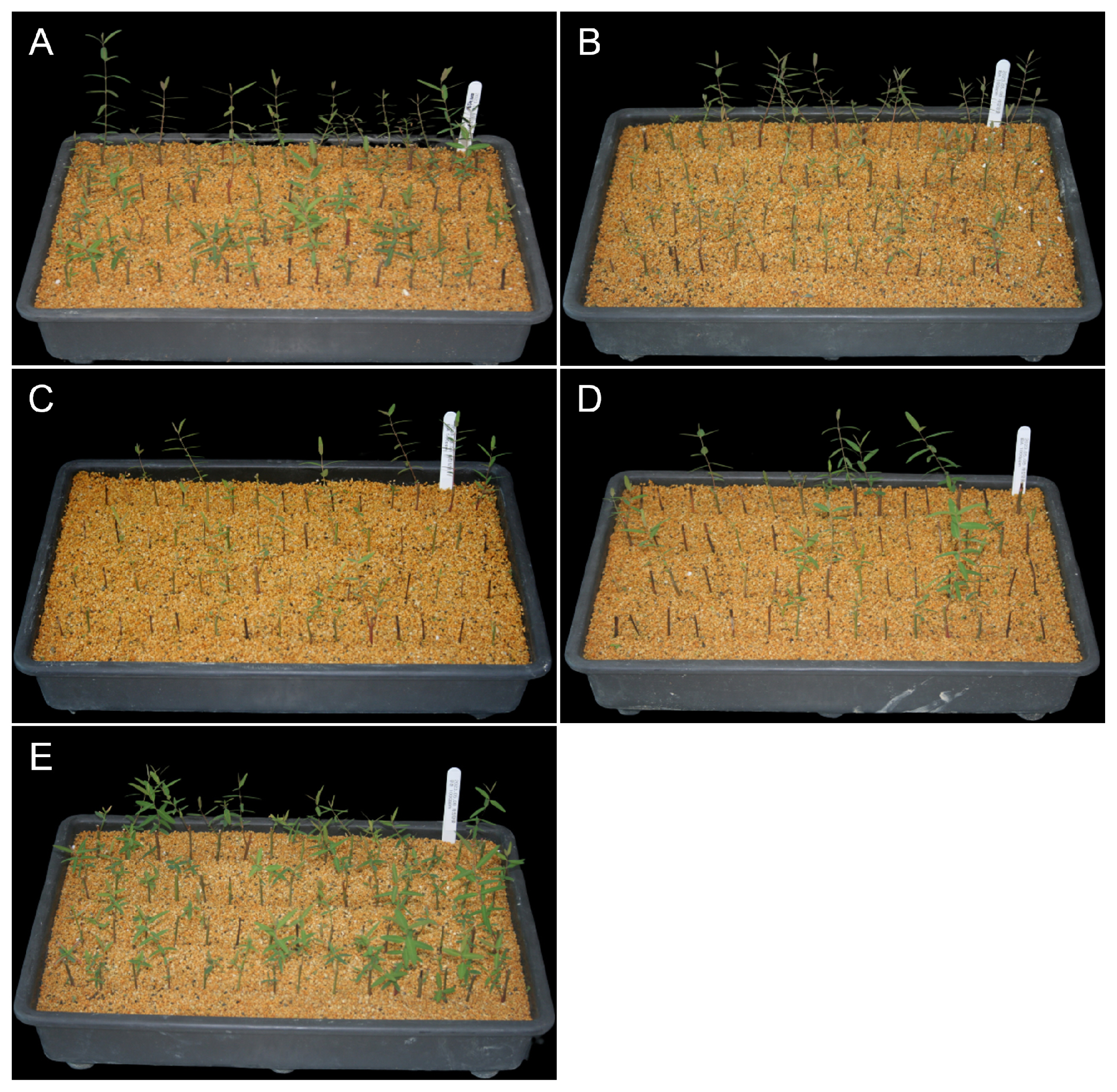
Among the known PGRs, auxin is known to facilitate adventitious rooting (Steffens and Rasmussen, 2016) and is widely used for the formation of adventitious roots in cutting propagation (Kochhar et al., 2008). Auxin is a tryptophan-derived hormone involved in promoting growth and development in most plants; additionally, it plays a role in plant gravitropism and phototropism (Woodward and Bartel, 2005; Kepinski and Leyser, 2005). These diverse effects across all plants arise from controlling cell division, elongation, and specific differentiation steps (Davies, 2004). Particularly, IBA is the most effective PGR in the formation of adventitious roots (Pop et al., 2011). Fig. 3 and Table 3 present the results of analyzing the effects of PGRs on A. lancifolium scions. The Rootone-treated treatment, control treatment, and 100 mg┬ĘLŌłÆ1 IBA treatment exhibited significantly higher cutting survival rates than the 500 mg┬ĘLŌłÆ1 and 1,000 mg┬ĘLŌłÆ1 IBA treatments. A similar pattern was found for rooting rates, with that for the Rootone-treated treatment reaching 86.67%. However, all treatments showed similar root lengths. Excessive auxin treatment can negatively affect root growth by increasing the production of ethylene (Burg and Burg, 1966). In this study, although there was no significant difference, lower cutting survival rates and rooting rates were observed in the 500 mg┬ĘLŌłÆ1 IBA treatment, which could partly be attributed to ethylene production. Conversely, the 1,000 mg┬ĘLŌłÆ1 IBA treatment showed the highest root number (9.33 ┬▒ 1.0). This indicated that treatment with 1,000 mg┬ĘLŌłÆ1 IBA led to an abundance of short roots (data not shown). The 1,000 mg┬ĘLŌłÆ1 IBA treatment also displayed the highest shoot height and the number of leaves, although these differences were not statistically significant. Further, auxin treatment did not seem to significantly affect the shoots of A. lancifolium scions.
To collect practical data on the cutting propagation of A. lancifolium, the effects of propagation media and PGRs on the scions were investigated. The cutting survival rate and rooting rate of A. lancifolium scions were the highest (both 81.7%) when Kanuma soil was used as the propagation medium. Contrastingly, the peat moss treatment showed a 0% rooting rate, indicating a lack of adventitious roots induction. The shoot height, the number of leaves, and dry weights of shoots and roots were the highest in the commercial potting mixes. When the cutting survival rate and rooting rate of scions were analyzed for different PGRs, the Rootone-treated treatment showed 10% higher survival rate and 5% rooting rate than the control treatment even though there was no statistical difference. Further, the treatment with high-concentration IBA increased the formation of roots but negatively affected the cutting survival rate and rooting rate. Overall, the results of this study are expected to be useful in establishing a mass production system for A. lancifolium via cutting propagation and in developing a method for advancing cultivar breeding.
Fig.┬Ā1
Preparation process of softwood cutting from 2-year-old Apocynum lancifolium. (A, B) Softwood cutting are taken from below a node. (C, E) The leaves are removed and the softwood cutting are 6 to 7 cm long. (F) The collected softwood was soaked in water.

Fig.┬Ā2
Effects of propagation media on rooting. (A) Kanuma soil, (B) commercial potting mixes, (C) perlite, and (D) peat moss. Bar = 10 cm.
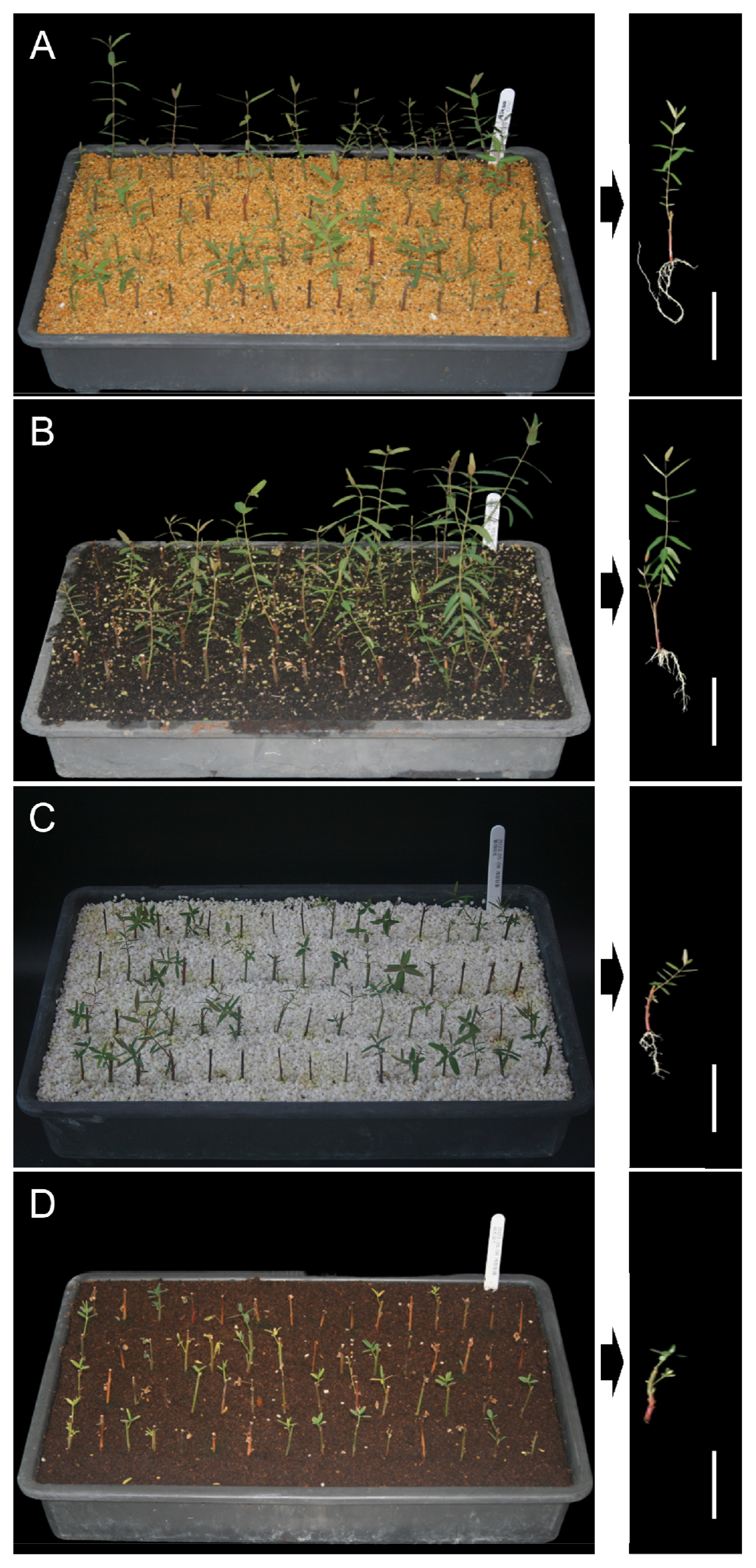
Fig.┬Ā3
Effects of plant growth regulators on rooting. (A) no treatment, (B) 100 mg┬ĘLŌłÆ1 IBA, (C) 500 mg┬ĘLŌłÆ1 IBA, (D) 1,000 mg┬ĘLŌłÆ1 IBA, (E) a 1:1 (v/v) solution of rootone (0.4% 1-naphthylacetamide) diluted in water.
Table┬Ā1
The rooting media properties
| Soil types | Components | pH | Manufacturer |
|---|---|---|---|
| Commercial potting mixes | coco peat, perlite, vermiculite, peat moss, zeolitez | 5.0 ŌĆō 6.0 | Hanareum. for Horticultural Nursery Substrate, Shinsung Co., Korea |
| Peat moss | 100% sphagnum peat moss | 4.0 | Sunshine® Mix #5, Sun Gro Horticulture, Inc., Agawam, MA, USA |
| Perlite | 100% perlite | 6.0 ŌĆō 7.0 | Pershine #1, GFC Co., Ltd, Korea |
| Kanuma soil | 100% fine-grained pumice | 5.0 ŌĆō 5.5 | Akagi Engei Co., Ltd., Japan |
Table┬Ā2
Effect of rooting media on the rooting and shooting of Apocynum lancifolium at 6 weeks after cuttings
| Rooting media | Survival rate (%) | Shoot height (mm) | The number of leaves | Rooting rate (%) | Root length (mm) | The number of roots | Dry weight (mg) | |
|---|---|---|---|---|---|---|---|---|
| Shoots | Roots | |||||||
| Commercial potting mixes | 40.0 ┬▒ 7.2 bz | 69.1 ┬▒ 14.3 a | 15.2 ┬▒ 1.1 a | 38.3 ┬▒6.9 b | 26.9 ┬▒ 2.3 b | 4.7 ┬▒ 0.6 a | 0.0819 ┬▒ 0.0098 a | 0.0054 ┬▒ 0.0008 a |
| Peat moss | 35.0 ┬▒ 6.3 b | 14.4 ┬▒ 1.8 b | 4.2 ┬▒ 0.8 c | 0.0 ┬▒ 0.0 c | 0.0 ┬▒ 0.0 c | 0.0 ┬▒ 0.0 b | 0.0045 ┬▒ 0.0012 b | 0.0000 ┬▒ 0.0000 b |
| Perlite | 66.7 ┬▒ 2.7 a | 29.9 ┬▒ 2.0 b | 9.4 ┬▒ 0.5 b | 53.3 ┬▒ 2.7 b | 19.8 ┬▒ 2.0 b | 3.9 ┬▒ 0.1 a | 0.0128 ┬▒ 0.0016 b | 0.0032 ┬▒ 0.0010 ab |
| Kanuma soil | 81.7 ┬▒ 5.0 a | 41.1 ┬▒ 5.1 ab | 10.5 ┬▒ 0.7 b | 81.7 ┬▒ 5.0 a | 36.2 ┬▒ 2.5 a | 4.6 ┬▒ 0.3 a | 0.0271 ┬▒ 0.0029 b | 0.0051 ┬▒ 0.0010 a |
| P values | 0.0001 | 0.0021 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0018 |
Table┬Ā3
Effect of auxin treatments on the rooting and shooting of Apocynum lancifolium at 6 weeks after cuttings
| Rooting media | Survival rate (%) | Shoot height (mm) | The number of leaves | Rooting rate (%) | Root length (mm) | The number of roots | Dry weight (mg) | |
|---|---|---|---|---|---|---|---|---|
| Shoots | Roots | |||||||
| Control | 81.7 ┬▒ 5.0 ay | 41.1 ┬▒ 5.1 | 10.5 ┬▒ 0.7 | 81.7 ┬▒ 5.0 a | 36.2 ┬▒ 2.5 | 4.6 ┬▒ 0.3 b | 0.0271 ┬▒ 0.0029 | 0.0051 ┬▒ 0.0010 |
| IBA 100 | 76.7 ┬▒ 4.3 a | 40.1 ┬▒ 4.7 | 10.6 ┬▒ 0.8 | 76.7 ┬▒ 4.3 a | 34.4 ┬▒ 1.7 | 5.9 ┬▒ 0.4 b | 0.0257 ┬▒ 0.0057 | 0.0091 ┬▒ 0.0047 |
| IBA 500 | 40.0 ┬▒ 4.7 b | 43.6 ┬▒ 3.7 | 10.8 ┬▒ 0.5 | 40.0 ┬▒ 4.7 b | 23.8 ┬▒ 2.0 | 6.0 ┬▒ 0.7 b | 0.0164 ┬▒ 0.0014 | 0.0037 ┬▒ 0.0005 |
| IBA 1000 | 31.7 ┬▒ 1.7 b | 53.1 ┬▒ 3.2 | 11.6 ┬▒ 0.3 | 31.7 ┬▒ 1.7 b | 32.1 ┬▒ 7.9 | 9.3 ┬▒ 1.0 a | 0.0215 ┬▒ 0.0010 | 0.0158 ┬▒ 0.0066 |
| Rootonez | 91.7 ┬▒ 6.3 a | 35.7 ┬▒ 2.6 | 9.8 ┬▒ 0.5 | 86.7 ┬▒ 6.1 a | 37.1 ┬▒ 2.6 | 4.9 ┬▒ 0.7 b | 0.0204 ┬▒ 0.0019 | 0.0040 ┬▒ 0.0007 |
| P values | 0.0001 | 0.0744 | 0.3130 | 0.0001 | 0.1960 | 0.0010 | 0.1540 | 0.1540 |
References
Anderson, V.L., R.A. McLean. 1974. Design of experiments NY, USA: Marcel Deckker Inc.
Burg, S.P., E.A. Burg. 1966. The interaction between auxin and ethylene and its role in plant growth. Proceedings of the National Academy of Sciences. 55:262-269.
https://doi.org/10.1073/pnas.55.2.262



Davies, P.J. 2004. Plant hormones: biosynthesis, signal transduction, action! Dordrecht, The Netherlands: Kluwer Academic Publishers.
Erstad, J.L.F., H.R. Gislerod. 1994. Water uptake of cuttings and stem pieces as affected by different anaerobic conditions in the rooting medium. Scientia Horticulturae. 58(1ŌĆō2):151-160.
https://doi.org/10.1016/0304-4238(94)90135-X

Fan, W., Y. Tezuka, Q. Xiong, M. Hattori, T. Namba, S. Kadota. 1999. Apocynins A-D: New phenylpropanoid substituted flavan-3-ols isolated from leaves of Apocynum venetum (Luobuma-Ye). Chemical and Pharmaceutical Bulletin. 47(7):1049-1050.
https://doi.org/10.1248/cpb.47.1049

Gruda, N., W.H. Schnitzler. 2004. Suitability of wood fiber substrate for production of vegetable transplants, I. Physical properties of wood fiber substrates. Scientia Horticulturae. 100(1ŌĆō4):309-322.
https://doi.org/10.1016/j.scienta.2003.10.001

Hartmann, H.T., D.E. Kester, F.T. Davies Jr, R.L. Geneve. 2011. Hartmann and KesterŌĆÖs plant propagation: Principles and practices (8th Ed.). Prentice Hall. Upper Saddle River, NJ:
Kepinski, S., O. Leyser. 2005. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 435:446-451.
https://doi.org/10.1038/nature03542


Kim, C.S., Z.S. Kim. 2012. Effects of cutting time, auxin treatment, and cutting position on rooting of the green-wood cuttings and growth characteristics of transplanted cuttings in the adult Prunus yedoensis
. Korean Journal of Horticultural Science and Technology. 30(2):129-136.
http://dx.doi.org/10.7235/hort.2012.11041

Kim, J.H., J.M. Lee, Y.G. Park, M.H. Chiang, J.A. Baik. 2021a. Analysis of free amino acids and antioxidant components by NaCl treatment of Korean native Apocynum lancifolium Russannov. Journal of Agricultural Life and Environmental Sciences. 33(3):299-310.
https://doi.org/10.22698/jales.20210029

Kim, J.H., Y.G. Park, S.W. Ann, J.A. Baik, D.J. Park. 2021b. Effects of salt treatment on seed germination and plant growth of Korean native Apocynum lancifolium Russannov. Journal of Environmental Science International. 30(11):957-965.
https://doi.org/10.5322/JESI.2021.30.11.957

Kochhar, S., S.P. Singh, V.K. Kochhar. 2008. Effect of auxins and associated biochemical changes during clonal propagation of the biofuel plant-Jatropha curcas
. Biomass and Bioenergy. 32:1136-1143.
https://doi.org/10.1016/j.biombioe.2008.02.014

Lee, J.G., B.Y. Lee. 2007. Effect of media composition on growth and rooting of highbush blueberry cuttings. Korean Journal of Horticultural Science and Technology. 25:355-359.
Lee, S.Y., N.H. Yoon, J.H. Gu, S.J. Jeong, K.J. Kim, J.C. Rhee, T.J. Lee, J.S. Lee. 2009. Effect of leaf number and rooting media on adventitious rooting of softwood cuttings in native Hydrangea serrata foracuminata
. Korean Journal of Horticultural Science and Technology. 27(3):199-204.
Oh, H.J., S.Y. Lee, U.S. Shin, H.C. Kim, S.Y. Kim. 2021. Several factors affecting growth of Veronica rotunda varsubintegra (Nakai) T. Yamaz. stem cutting. Korean Journal of Plant Research. 34(4):270-277.
https://doi.org/10.7732/kjpr.2021.34.4.270

Pop, T.I., D. Pamfil, D. Bellini. 2011. Auxin control on the formation of adventitious roots. Notulae Botanicae Horti Agrobotanici Cluj-napoca. 39:307-316.
https://doi.org/10.15835/nbha3916101

Son, S.W., B.C. Lee, H.H. Yang, Y.J. Seol. 2011. Distribution of five rare plants in Korea. Korean Journal of Plant Taxonomy. 41:280-286.

Steffens, B., A. Rasmussen. 2016. The physiology of adventitious roots. Plant Physiology. 170:603-617.
https://doi.org/10.1104/pp.15.01360


Verdonck, O., P. Demeyer. 2004. The influence of the particle sizes on the physical properties of growing media. Acta Horticulturae. 344:99-101.
https://doi.org/10.17660/ActaHortic.2004.644.10

Walternberger, B., A. Mocan, K. Smeijkal, E.H. Heiss, A.G. Atanasov. 2016. Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules. 21:807-830.
https://doi.org/10.3390/molecules21060807



Woodward, A.W., B. Bartel. 2005. Auxin: regulation, action, and interaction. Annals of Botany. 95(5):707-735.
https://doi.org/10.1093/aob/mci083



Zheng, M., C. Liu, F. Pan, D. Shi, Y. Zhang. 2012. Antidepressant-like effect of hyperoside isolated from Apocynum venetum leaves: Possible cellular mechanisms. Phytomedicine. 19:145-149.
https://doi.org/10.1016/j.phymed.2011.06.029








