 |
 |
- Search
| J. People Plants Environ > Volume 26(1); 2023 > Article |
|
ABSTRACT
Background and objective: The objective of this study was to produce Asiatic hybrid lily with more than two flowers in one year starting from bulbils. This study was to force ‘Beni no Mai’ bulbils influenced by harvesting date, flower bud removal, and temperature manipulation and to evaluate dormancy and maturity by nuclear magnetic resonance imaging (NMRI) and carbohydrates analysis.
Methods: In experiment 1, bulbils were harvested at 0–30 days after anthesis (DAA), and were divided into 7 stages. And, soluble monosaccharides and monosaccharide constituents of bulbils were analyzed. In experiment 2, flower buds of plants that flowered on 21 April were not removed or removed on 1, 13, and 25 April. Bulbils harvested were divided into four groups and planted after cold treatment. In experiment 3, bulbils received a sequential temperature (SEQ CD) treatments for three weeks at 2.5, 5.0, and 7.5°C, 1 week at 10, 12.5, and 15°C, and 3 weeks at 2.5, 5.0, and 7.5°C. In experiment 4, bulbils were harvested 0–70 DAA, and were subjected to spin-spin lattice relaxation time by NMRI.
Results: The bulbils harvested at 30 DAA produced two flowers within 282 days, and were better in growth. The bulbils harvested at 30 DAA showed higher concentration of soluble glucose and lower concentration of non-cellulosic neutral glucose. Growth of bulbils removed flower buds before flowering were better than those of bulbils that flower bud was removed at anthesis and 13 Apr. However, the number of flowers of bulbils harvested from plants removed flower buds on 1 Apr. were less than 2. The SEQ CD treatment of 7.5°C/3W–15°C/1W–5°C/3W in bulbils could produce the plants with 2.2 flowers within 300 days after planting. When bulbils were harvested at 0–28 DAA, yellow color corresponding to T2 relaxation time of 15–20 ms was dominant, and bulbils harvested at 56–70 DAA showed T2 relaxation time of 35–50 ms and 50–100 ms.
Conclusion: Considering the results of soluble carbohydrate content and T2 relaxation time of bulbils, it was found that the bulbils had a shallower dormancy and were more mature at 30–42 DAA than before 28 DAA. Also, when these bulbils were planted after SEQ CD treatment at 7.5°C/3W–15°C/1W–5°C/3W, plants with two or more flowers could be produced within one year.
Methods: In experiment 1, bulbils were harvested at 0–30 days after anthesis (DAA), and were divided into 7 stages. And, soluble monosaccharides and monosaccharide constituents of bulbils were analyzed. In experiment 2, flower buds of plants that flowered on 21 April were not removed or removed on 1, 13, and 25 April. Bulbils harvested were divided into four groups and planted after cold treatment. In experiment 3, bulbils received a sequential temperature (SEQ CD) treatments for three weeks at 2.5, 5.0, and 7.5°C, 1 week at 10, 12.5, and 15°C, and 3 weeks at 2.5, 5.0, and 7.5°C. In experiment 4, bulbils were harvested 0–70 DAA, and were subjected to spin-spin lattice relaxation time by NMRI.
Results: The bulbils harvested at 30 DAA produced two flowers within 282 days, and were better in growth. The bulbils harvested at 30 DAA showed higher concentration of soluble glucose and lower concentration of non-cellulosic neutral glucose. Growth of bulbils removed flower buds before flowering were better than those of bulbils that flower bud was removed at anthesis and 13 Apr. However, the number of flowers of bulbils harvested from plants removed flower buds on 1 Apr. were less than 2. The SEQ CD treatment of 7.5°C/3W–15°C/1W–5°C/3W in bulbils could produce the plants with 2.2 flowers within 300 days after planting. When bulbils were harvested at 0–28 DAA, yellow color corresponding to T2 relaxation time of 15–20 ms was dominant, and bulbils harvested at 56–70 DAA showed T2 relaxation time of 35–50 ms and 50–100 ms.
Conclusion: Considering the results of soluble carbohydrate content and T2 relaxation time of bulbils, it was found that the bulbils had a shallower dormancy and were more mature at 30–42 DAA than before 28 DAA. Also, when these bulbils were planted after SEQ CD treatment at 7.5°C/3W–15°C/1W–5°C/3W, plants with two or more flowers could be produced within one year.
Two to three years are usually required to produce the Easter lily (Lilium longiflorum Thunb.) bulbs (Roberts et al., 1985) or at least two years for the Asiatic hybrid lily (Lilium × elegans Thunb.) from scaling (Roh, 1996) for commercial forcing in the greenhouse. Only a limited number of commercially available Asiatic hybrid lily cultivars have been propagated by leaf cuttings (Roh, 1988). The technique reported for forcing of leaf-cutting propagated bulblets of the Easter lily (Oglevee et al., 1986; Roh, 1982) is, therefore, not suitable for forcing Asiatic hybrid lilies.
Therefore, bulbils that are naturally forming at the leaf axils in several Asiatic hybrid lilies should be used as a propagule for forcing. When bulbils are used for forcing, bulb production phase and programming phase is combined, eliminating the bulb production phase (Roh, 1996, 2011). This will enable combining the programming phase to induce flower bud initiation and the first stage of forcing from shoot emergence to flower bud initiation, and thus the total production time can be shortened (Roh, 1996). The Asiatic hybrid ‘Beni no Mai’ that produces bulbils in the leaf axils at flowering can produce one to two flowers in less than a year when mature bulbils are subjected to a SEQ CD treatment of 14 to 20 days each at 5°C–15°C and 20°C–5°C (Roh, 1992; Suh and Roh, 2014).
Dormancy and maturity of the Tiger lily (L. lancifolium Thunb.) was studied, and five to seven flowers per plant have been produced in two years when programmed bulbils were forced (Roh, 1978a, 1978b). In other Asiatic hybrid lilies, only one flower has been produced from bulbs weighing 10 g after field production or two flowers forcing bulbs of ‘Rouge’ weighing 4 g (Zhang et al., 1990).
Dormancy in scales of L. longiflorum, L. callosum and their interspecific hybrids (Roh et al., 1996) has evaluated by spin-spin lattice relaxation time (T2 relaxation time) of water molecules. Lilium longiflorum which has shallow dormancy has a longer T2 relaxation time than that of L. callosum which has a deep dormancy and that T2 relaxation time of their interspecific hybrids was intermediate. The level of bud dormancy has been evaluated for apple (Faust et al., 1991) and blueberry (Vaccinium corymbosum L. (Rowland et al., 1992). Application of 1H-nuclear magnetic resonance imaging (NMRI) analysis has been successfully performed to image water status in tissues of barley (Hordeum vulgare L. cv. New Golden), soybean (Glycine max Merr. cv. Enrei) (Kano et al., 1990), and apple (Millard et al., 1993) to understand the dormancy and water status. However, studies that analyze or attempt to understand the physiological nature of dormancy and maturity using NMRI in small bulbils that should be grown for at least two years to reach bulbs to force to flower have not been reported yet.
The growing period starting from bulbils to flower could be divided into two phases. The first phase is from potting treated bulbils to shoot emergence that lasts about 200 to 230 days. The second phase is from shoot emergence to flowering that lasts about 100 days (Roh, 1996, 2011; Suh and Roh, 2014). Dormancy and maturity or immaturity of large Easter lily bulbs compared to bulbils of L. lancifolium have been well discussed (Roh and Wilkins, 1977a, 1977b), including delayed shoot emergence of dormant bulbs and increased number of flower buds as a criterion for maturity. In addition, maturity can be induced from immature bulbs in Lilium longiflorum (Roh and Wilkins, 1977a, 1977b) and Ornithogalum thyrsoides Jacq. (Roh et al., 2007). Bulbils of ‘Beni no Mai’ have been harvested 40–50 DAA. However, induction of maturity of bulbils by manipulating the time of harvest and removal of flower buds has not been reported yet. Furthermore, T2 relaxation time as affected by time of bulbil harvest has not been investigated yet, although the internal structure during fruit development by MRI and seed germination of Styrax japonicus Sieb. et Zucc. have been well documented (Horimoto et al., 2011; Roh et al., 2004).
The objectives of this research were to evaluate the growth and flowering of ‘Beni no Mai’ bulbils influenced by harvesting date, flower bud removal, and temperature manipulation, to study the T2 relaxation time analyzed by NMRI of bulbils as affected by bulbil harvest time to understand dormancy and maturity, and to evaluate changes of soluble sugars and non-cellulosic neutral sugars of cell walls.
Asiatic hybrid lily ‘Beni no Mai’ bulbs (14–16 cm in circumference) were planted after 8 weeks of 5°C treatment and grown in growth chambers as specified in each experiment or in a greenhouse maintained at 21/16°C (day/night) except at 25–30/22–26°C (day/night) from June to September. Bulbils formed in the middle of the stem or other parts of the stem as indicated in each experiment were collected and packed with peat moss (60% moisture) in polyethylene bags. Bulbils that formed scaly leaves or split into two bulbils like a double nose bulb (Roh and Wilkins, 1977c) were excluded from evaluation (Fig. 1). These experiments were conducted following the general cultural information described previously (Suh and Roh, 2014).
After the respective temperature treatments described in each experiment, one bulbil was planted in a 10 cm pot following cultural practices as described previously (Suh and Roh, 2014). Two weeks after potting, 1 g of 14N-6P-11.7K slow-release fertilizer (Scott Co., Marysville, OH, USA) was applied to the surface of the growing medium. A liquid feeding at 200 mg·L−1 N using 20N-8.7P-16.7K Peter’s water-soluble fertilizer (Grace-Sierra, Allentown, PA, USA) was applied once a month.
Bulbils from 20 stock plants of ‘Beni no Mai’ that flowered between 20 April and 22 April were harvested at 0 (anthesis), 10, 20, and 30 DAA. Bulbils were grouped according to the size or color ranging from green-yellow-brown and less than 2 mm in diameter at anthesis (0 DAA; stage 1) to bulbils that had formed scaly leaves with dark brown scales ( 30 DAA; stage 6 and 7).
Weights of randomly selected 10 bulbils were recorded in triplicate to obtain the average weight of individual bulbil after bulbil harvest. After SEQ CD treatment at 5°C-20°C-5°C for 14 days each, individual bulbil was planted in 10 cm pot and greenhouse forcing was carried out. During greenhouse forcing, all pots were completely randomized. Number of flowered plants and days to flowering were counted from potting the bulbil. At flowering, number of flowers and leaves and plant height were recorded.
Soluble monosaccharides and monosaccharide constituents of non-cellulosic neutral sugars of cell walls of bulbils that received SEQ CD treatment were analyzed in triplicates. In brief, 4 g of bulbils were placed in 16 mL of 80% ethanol and a 1 mL of aliquot was analyzed following published methods (Gross, 1983; Li and Schuhmann, 1980). Monosaccharide constituents of non-cellulosic neutral sugars were quantified in triplicates after hydrolyzation with 2 M trifluoroacetic acid. Monosaccharides were analyzed as described by Gross (1983) and Gross et al. (1986).
Flower buds of plants of ‘Beni no Mai’ that flowered on 21 April were not removed or removed on 1, 13, and 25 April Bulbils that harvested on 24 Mays were divided into four groups of large, medium, small, and extra small size. Weights from 10 bulbils in a group were recorded and replicated 4 times. An then, bulbils were treated for six weeks at 5 °C, planted in 10 cm pots, and forced to flower. Growth was recorded as in the previous experiment.
From plants of ‘Beni no Mai’ that flowered on 25 April, bulbils (average weight 539 mg of individual bulbil) harvested on 26 May. Bulbils were packed in peatmoss received SEQ CD treatments for three weeks at 2.5, 5.0, and 7.5 °C (factor A), for one week at 10, 12.5, and 15 °C (factor B), and for three weeks at 2.5, 5.0, and 7.5 °C (factor C) for a total of 27 SEQ CD treatments. Bulbils were potted on 14 July and grown in the greenhouse. There were 15 plants per treatment and growth was recorded as in the previous experiment.
From plants of ‘Beni no Mai’ that flowered on 23 April, inflorescences were cut-off below bracts. Bulbils were collected from the mid-position of stem 0, 14, 28, 42, 56, and 70 DAA. Weights of bulbils were recorded for each harvest date. For NMRI, bulbils were placed into a 25 mm NMR tube and image slices were taken as described previously (Millard et al.,1993; Roh et al., 2004). For imaging, a Bruker 400 MHz (9.4-T) NMRI instrument (Bruker Instrument Co., Billerica, MA, USA) was used. Six bulbils were imaged for each harvest date and only the representing images are presented.
Distribution percentages of T2 relaxation time of total images for T2 relaxation time between 0 and 55 milliseconds (ms) were plotted. The area of trapezium (TA or TA’) was calculated with a distance (a) corresponding to T2 relaxation time at 30 ms and 2% distribution percentage and percentage of total distribution at T2 relaxation time at 50 ms (b) with 20 ms difference as a fixed constant. Further, T2 relaxation time corresponding to the highest peak of total distribution was compared whether T2 relaxation time was shorter (−) or longer (+) as compared to T2 relaxation time of 20 ms that was arbitrarily selected based on the distribution of all samples.
Weights of bulbils harvested 10 DAA (stage 4) were 232 mg. Bulbils of stage 1–3 weighed less than 120 mg (Table 1). Bulbils harvested 20 DAA (stage 5) and 30 DAA (stage 6) weighed 351 mg and 342 mg, and flowered in 286 and 285 days, respectively, showing no significant difference between the two harvest dates. However, the number of flowers from bulbils harvested at 30 DAA was 1.9–2.0, which was significantly higher than that of bulbils harvested at 20 DAA. Plant height and the number of leaves of bulbils collected at stages 5, 6 and 7 were not significantly different from each other. Therefore, it was judged that it is desirable to plant after harvesting bulbils on 30 DAA considering the number of flowers and shoot growth of ‘Beni no Mai’, (stage 6 and 7).
Concentrations of soluble fructose and sucrose were higher when bulbils were harvested 20 DAA than at anthesis and 10 DAA, reaching 0.98 and 1.93 mg/g FW, respectively (Table 2). Soluble glucose concentrations was the highest (1.77 to 2.00 mg/g·fw), when bulbils were harvested at 30 DAA (stage 7). Non-cellulosic neutral monosaccharides of cell wall component decreased steadily as bulbil harvest date was delayed after anthesis except for bulbils that formed scaly leaves at harvest (stage 7). Glucose concentration was decreased significantly from 24.57 mg/g FW when bulbils were harvested 0 DAA to 1.82 mg/g FW when bulbils were harvested 30 DAA (stage 7).
Weights of bulbils were significantly affected by flower bud removal date (Table 3). Weights of bulbils harvested on 1 Apr. from plants when flower buds were removed were generally heavier than those of bulbils that flower bud were removed after 13 April. Large and medium size bulbils harvested from plants after flower bud removal on 1 April (at anthesis) were 501 and 363 mg, respectively. And large size bulb harvested from plants with flower bud removed on 13 April was 348 mg. Weights of bulbils were less than 300 mg when flower buds were removed on 25 April or not removed regardless of bulbil size.
The days to shoot emergence and flowering ranged from 209 to 214 days and from 293 to 299 days, respectively, regardless of flower bud removal dates and sizes of bulbils. The highest number of flowers (1.6) was obtained when large bulbils were harvested from plants with flower bud removed on 1 April (Table 3). Medium, small and extra small bulbils regardless of flower bud removal produced less than 1.4 flowers. Plant height ranged from 44 cm and 52 cm and was longer as the bulbil size increased regardless of the flower bud removal dates. The number of leaves varied greatly, ranging from 30 (small and extra small bulbils of no bud removal and extra small bulbils of bud removal on 13 and 25 April to 45 (large bulbs when flower buds were removed on 1 April The number of leaves were significantly higher in the treatment removed flower bud on 1 April than in the no flower bud removal treatment. Therefore, these results suggested that planting after harvesting large bulbils by rapid flower bud removal was effective for the number of flowers and growth of ‘Beni no Mai’, but the number of flowers were less than 2.
When bulbils received SEQ CD treatments with intercalation for one week at 10, 12.5, and 15°C after 3 weeks of 2.5, 5, and 7.5°C given for a total of 6 weeks (2.5, 5, and 7.5°C /3W–10, 12.5, and 15°C/1W–2.5, 5, and 7.5°C/3W), shoot emerged in 202 (7.5°C/3W–15°C/1W–5°C/3W) and 208 days (5°C/3W–12.5°C/1W–2.5°C/3W) and flowered in 299 (7.5°C/3W–12.5°C/1W–5°C/3W) and 305 days (2.5 or 5°C/3W–12.5°C/1W–2.5 or 5°C/3W) (Table 4). The number of flowers were the smallest with 1.8 in the 5°C/3W–10°C/1W–2.5°C/3W and 2.5°C/3W–12.5°C/1W–5°C /3W treatments, and the highest with 2.2 in 7.5°C/3W–15°C/1W–5°C/3W treatment. Plant height ranged from 57 to 74 cm, and was the longest at 74 cm in 7.5°C/3W–15°C/1W–5°C/3W treatment. The number of leaves ranged from 49 to 70, and were higher with 68–70 in 7.5°C/3W–15°C/1W–5°C/3W and 7.5°C/3W–12.5°C/1W–7.5°C/3W treatments than the other treatments. Therefore, 7.5°C/3W–15°C/1W–5°C/3W treatment of bulbils was the most effective for many flowers and good shoot growth in ‘Beni no Mai’.
Weights of bulbils continued to increase from 138 mg when harvested at 0 DAA to 433 mg at 42 DAA and 754 mg at 70 DAA (Table 5). T2 relaxation time across the cross section of a bulbil and stem harvest at 0 DAA ranged from, depending on the position of scales, 15–20 ms (yellow color) for 3–4 scales counted from outside. It was partly 30–35 ms for purplish-red or maroon in outermost scales. However, stems showed dominantly green (25–30 ms) with scattered purplish red (30–35 ms) (Fig. 2).
When harvest dates were delayed to 14 DAA and 28 DAA, T2 relaxation time of stem corresponding to 30–35 ms (purplish red) became evident, showing clear ring while inside and outside of this ring were green with T2 relaxation time of 25–30 ms. During these stages, yellow color corresponding to T2 relaxation time of 15–20 ms became dominant in bulbils, although purplish red with T2 relaxation time of 35–40 ms started to become evident. Bulbils harvested at 42 DAA showed transient T2 relaxation time between those harvested earlier and those harvested later at 56 DAA.
In cross sections of the entire stem when bulbils were harvested at 0, 14, 24 (full cross section of stem), and 42 DAA (a partial section), areas of green with 25–30 ms T2 relaxation time were gradually reduced while dark purple red with 30–35 ms T2 relaxation time and pink with 35–50 ms T2 relaxation time increased. As bulbils were easily separated from the stem at 56 DAA, stems were not included in bulbils collected at 56 or 70 DAA (Fig. 2). Bulbils harvested especially at 56 and 70 DAA showed T2 relaxation time of 35–50 ms and 50–100 ms, predominately 50–100 ms, especially for scale positions S3–S5. Scale S1 did not show T2 relaxation time longer than 30–35 ms.
Distribution percentages of T2 relaxation time of total images of each individual shorter than 55 ms were plotted (Fig. 3). Increase in T2 relaxation time as bulbil harvest dates were delayed was presented by the area of trapezium with the base of 2% distribution corresponding to 30 ms and 50 ms. Trapezium area (percentage of total image × ms) for bulbils harvested at anthesis and 70 DAA was increased from 0.22 (TA) to 0.83 (TA’) (Table 5, Fig. 3). Furthermore, relaxation distribution histogram from the cross section of bulbils peaked between 17 and 24 ms based on the T2 at 20 ms when the peak position of T2 relaxation time was compared. If peaks were shorter than 20 ms, negative values were assigned: −2.67 ms based on 20 ms for bulbils harvested at anthesis which was increased to + 1.16 ms and + 3.16 ms for bulbils harvested at 56 and 70 DAA, respectively. Bulbils harvested at 42 DAA showed T2 relaxation time of −0.44 ms, close to the baseline of 20 ms.
Based on weights of bulbils and flowering responses, especially focusing on the number of flowers, bulbils harvested 30 DAA (stage 6) were considered mature, earlier than the previous report (40–50 DAA) (Suh and Roh, 2014). Therefore, bulbils harvested 30 DAA weighing about 340 mg produced a significantly high number of flowers (1.9–2.0 flowers).
Although there is no report on translocation of carbohydrates from leaves to developing bulbils attached to leaf axils, increase in soluble carbohydrates could indicate that bulbils are maturing, perhaps due to an increase in translocation from leaves and shoot. Carbon metabolism has been investigated in detail in whole Lilium longiflorum Thunb. And scale filling of bulb was promoted when assimilates were translocated from leaves to scales (Wang, 1981). Bulbils may compete as a sink competing with scales during enlargement from bulbil to bulblet formation, but could preferably receive assimilates from leaves as bulbils are attached to leaves.
Decreases of all neutral sugars of cell wall components could indicate that bulbil is maturing, losing most of neutral sugars in cell walls because of solubilization of poly-matrix components during ripening of fruits (Gross and Wallner, 1979; Rose et al., 1998). Considering changes in free and cell wall neutral sugars, maturity is considered to be related to the increase and decrease of sugars, respectively. Weight of mature bulbils could be increased, and more flowers are expected to be produced as compared to immature bulbils due to an induction of maturity from immature bulbs, especially following SEQ CD treatment (Roh and Wilkins, 1977c). Bulbils harvested at 30 DAA (stage 6 and 7) were considered mature based on flowering responses, i. e., early flowering and increased number of flowers.
Therefore, in this study, it was found that bulbils of ‘Beni no Mai’ matured on 30 DAA through carbohydrate analysis. And it was determined that a plants with two flowers could be produced within one year if these mature bulbils were planted.
In Paeonia lactiflora, the root dry weight and number of roots increased when flower buds were removed prior to flowering compared to full flowering (Kim et al., 1998). Also, earlier flower bud removal increased number of roots, root fresh weight, and rhizome yield in Atractylodes japonica (Park et al., 2004). These reports indicated that the flower bud removal time was an important factor for root growth, and that rapid flower bud removal was effective for root growth. For tulips, which are bulbous plants such as lilies, flower bud removal also increased the yield of bulbs (Miao et al., 2014). The flower bud removal was to prevent the consumption of nutrients required for flowering, and then these nutrients migrated to bulbs promoted the growth of bulbs. In this study, large-sized bulbils (501 mg) of Asiatic hybrid lily ‘Beni no Mai’ could be harvested by flower bud removal on April 1, 20 days before flowering, and planting these bulbils could produce plant with 1.6 flowers.
Although shoot emergence and flowering dates were significantly affected by temperature treatments, the difference was only 6 days, which might not be meaningful when shoot emergence and flowering took longer than 202 and 299 days, respectively, from planting bulbils. With SEQ CD treatments, it was clear that about two flowers could be produced for bulbils of ‘Beni no Mai’ with the maximum of 2.2 flowers with 7.5°C/3W–15°C/1W–5°C/3W SEQ CD treatment, different from the SEQ CD temperatures previously reported (Suh and Roh, 2014).
As these SEQ CD treatments were given at least 200 days prior to shoot emergence and 300 days prior to flowering, further studies should be carried out to investigate other environment factors during the growth period of scaly leaf formation and development of bulblets after planting treated bulbils. There are no physiological parameters to understand whether bulbils are immature or mature upon harvest, except for the evaluation based on leaf emergence and flowering two years later responding to temperature and plant growth regulators as investigated for bulbils of L. lancifolium Thunb. (Roh, 1978a, b).
Bulbils are morphologically simpler structures (Fig. 1) than large Easter lily bulbs composed of mother scales and daughter scales. Therefore, it is not feasible to study the translocation of maturity factors that control flowering and flower numbers as studied for double nose of Easter lily bulbs (Roh and Wilkins, 1977c). Levels of dormancy in L. longiflorum, L. callosum, and their interspecific hybrids have been evaluated by MRI (Roh et al., 1996). Thus, bulbils harvested from different stages of development were subjected to MRI to understand their immaturity and maturity.
Physiology of dormancy and maturity of small bulbils used as propagules was not understood. NMRI has been used as a method to determine the degree of dormancy by nondestructively measuring the moisture states (T2 relaxation time) of plants (Liu et al., 1993). T2 relaxation time could also be used to understand the level of dormancy in scales of L. callosum, L. longiflorum Thunb. and their interspecific hybrids (Roh et al., 1996) and could be used to determine the level of maturity. Therefore, T2 relaxation time was analyzed to study the dormancy and its possible relationship with maturity in bulbils of ‘Beni no Mai’. Bulbils of ‘Beni no Mai’ harvested at 0, 14 and 28 DAA were considered immature based on flowering data and a previous report (Suh and Roh, 2014), and these bulbils showed T2 relaxation time of 15–20 ms (yellow color).
Bulbils harvested at 42 DAA showed transient T2 relaxation time between immature bulbils harvested earlier and mature bulbils harvested later. The T2 relaxation time of bulbils harvested at 42 DAA was changing from 15–20 ms to 30–35 ms. On the other hand, bulbils harvested at 56 and 70 DAA showed T2 relaxation time of 35–50 ms and 50–100ms. When bulbils are harvested late after anthesis (at 56 and 70 DAD), water is considered relatively free with long T2 relaxation time as compared to bulbils harvested prior to 42 DAA, suggesting that bulbils are released from dormancy, such as apple buds with T2 relaxation time up to 30 ms ( Faust et al., 1991; Liu et al., 1993).
The bulbils harvested at 30 DAA produced two flowers within 282 days, which was significantly higher than those of bulbils harvested at anthesis, 10, and 20 DAA. Also, plant height and the number of leaves of bulbils collected at 30 DAA were significantly different from those harvested at anthesis and 10 DAA. Therefore, it was judged that it is desirable to plant after harvesting bulbils on 30 DAA considering the number of flowers and shoot growth of ‘Beni no Mai’ (stage 6 and 7).
As the harvest date of bulbils was delayed after flowering, the concentration of soluble glucose increased, and concentration of non-cellulosic neutral glucose decreased. The bulbils harvested at 30 DAA showed higher concentration of soluble glucose and lower concentration of noncellulosic neutral glucose than bulbils harvested at anthesis, 10 and 20 DAA. Therefore, it was indicated that the changes in glucose concentration could be used as an indicator of the maturity of the bulbils.
Weights of bulbils harvested from plants removed flower buds on 1 Apr. before flowering were generally heavier than those of bulbils that flower bud were removed at anthesis and 13 Apr. Also, plant height and number of leaves were higher in bulbils harvested in the treatment with early flower bud removal. However, the number of flowers of bulbils harvested from plants removed flower buds on 1 Apr. were less than 2.
The emergence of shoot and flower was the fastest in SEQ CD treatments of 7.5°C/3W–15°C/1W–5°C/3W and 7.5°C/3W–12.5°C/1W–5°C/3W, respectively. The number of flowers were the highest with 2.2 and plant height was the longest at 74 cm in 7.5°C/3W–15°C/1W–5°C/3W treatment. Therefore, it was indicated that SEQ CD treatment of 7.5°C/3W–15°C/1W–5°C/3W in bulbils could produce the plants with 2.2 flowers of 74 cm in length within 300 days after planting.
When bulbils of ‘Beni no Mai’ were harvested at 0, 14, and 28 DAA, yellow color corresponding to T2 relaxation time of 15–20 ms was dominant. The T2 relaxation time of bulbils harvested at 42 DAA was changing from 15–20 ms to 30–35 ms. On the other hand, bulbils harvested at 56 and 70 DAA showed T2 relaxation time of 35–50 ms and 50–100ms. These results showed that the bulbils had a shallow dormancy after 42 DAA.
In this study, considering the results of soluble carbohydrate content and T2 relaxation time of bulbils, it was found that the bulbils had a shallower dormancy and were more mature at 30–42 DAA than before 28 DAA. Also, when these bulbils were planted after SEQ CD treatment at 7.5°C/3W–15°C/1W–5°C/3W, plants with two or more flowers could be produced within one year.
Fig. 1
Bulbils of Lilium ×elegans ‘Beni no Mai’ hybrid lily with different morphologies. Only bulbils with morphology 1 were used and those with scaly leaves (2), double-nose bulb like double-bulbils (3), or double-nose bulb like double-bulbils with scaly leaves (4, 5) were not used in the experiment. Bulbil 1 were used in experiment, and bulbils with scaly leaves and double-bulbils were not used in experiment. Bar = 5 mm.

Fig. 2
NMRI. Images of T2 relaxation time of cross section of Lilium ×elegans ‘Beni no Mai’ lily bulbils and stem as influenced by bulbil harvest time. T2 relaxation times are color coded from dark red ( < 10 ms) to purple (51 – 100 ms). Bulbil was attached to the leaf axil when bulbils were collected at 0 (A), 14 (B), 28 (C) days after anthesis, and separated in 4 bulbils when harvested at 42 (D) days after anthesis, and all harvested at 56 (E) and 70 (F) days after anthesis were separated.

Fig. 3
NMRI distribution percentage of T2 relaxation time of total images bulbils harvested at anthesis (A) and 70 DAA (B). The area (percentage of total image × ms) of trapezium (TA or TA’) was calculated with a distance (a) corresponding to T2 relaxation time at 30 ms and 2% distribution percentage and percentage of total distribution percent at T2 relaxation time at 50 ms (b) with 20 ms as a fixed constant (frame 70). Further, the position of highest peak at T2 relaxation time was compared to the peak at T2 relaxation.
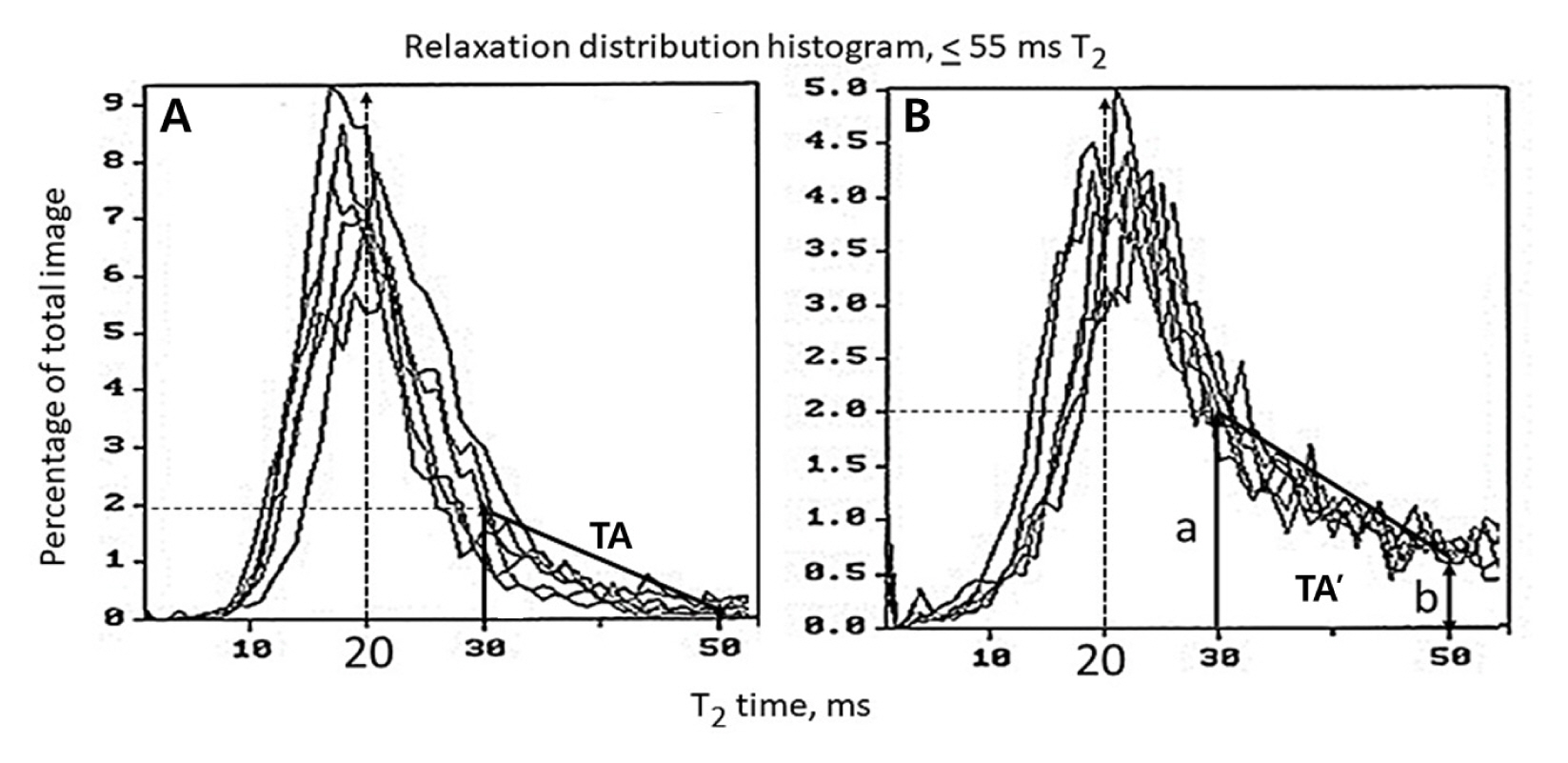
Table 1
Description of bulbils and growth and flowering of ‘Beni no Mai’ Lilium ×elegans lily bulbils as influenced by stages and harvest dates
| Harvest dates of bulbils and stages | Description of bulbils and positions/developmental stages of bulbils at harvest | Weight of bulbil (mg) | Days to flowering | Flowering (%)z | No. of flowers | Plant height (cm) | No. of leaves |
|---|---|---|---|---|---|---|---|
| At anthesis (stage 1) | Less than 2 mm in diameter, mainly from near the bract, mostly green-yellow-brown. | 25 fy | 319 a | 20 | 1.0 d | 51 d | 38 d |
| At anthesis (stage 2) | Bulbils formed 5–6 leaves below the bracts: bulbils, 3–5 mm in diameter. Upper part of bulbils turns to light brown. | 61 e | 303 b | 20 | 1.0 d | 56 b | 47 c |
| At anthesis (stage 3) | Bulbils formed 10–15 leaves below the bracts; bulbils, 5 mm in diameter, upper part of bulbils turns to brown. | 120 d | 298 c | 53 | 1.1 d | 54 c | 52 ab |
| 10 DAA (stage 4) | Bulbils formed 10–15 leaves below the bracts: bulbils, 5–6 mm in diameter. Previously called immature bulbs. | 232 c | 289 d | 53 | 1.2 c | 55 bc | 54 ab |
| 20 DAA (stage 5) | Bulbils formed 15–20 leaves below the bracts: bulbils, 6–7 mm in diameter. Collected 20 DAA. Previously called immature | 351 a | 286 de | 87 | 1.4 b | 58 a | 55 a |
| 30 DAA (stage 6) | Bulbils formed 15–20 leaves below the bracts: bulbils, 7–8 mm in diameter. Previously called mature bulbils. | 342 ab | 285 de | 93 | 1.9 a | 58 a | 57 a |
| 30 DAA (stage 7) | Bulbils formed 10–15 leaves below the bracts. Sprouting 30 DAA. Scaly leaves are green while scales are dark brown. | 334 b | 282 e | 93 | 2.0 a | 58 a | 55 a |
Table 2
Soluble monosaccharides and monosaccharide constituents of non-cellulosic polysaccharides of the cell wall components of bulbils of ‘Beni no Mai’ Lilium ×elegans lily harvested at different stages of development
| Harvest dates of bulbils and stagesz | Soluble monosaccharides (mg/g FW) | Non-cellulosic neutral monosaccharides of cell wall (mg/g FW) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Fructose | Glucose | Sucrose | Rhamnose | Arabinose | Xylose | Mannose | Glucose | Galactose | |
| At anthesis (stage 1) | 0.72 fy | 0.86 f | 0.93 e | 0.59 a | 1.74 a | 0.58 a | 1.83 a | 24.57 a | 1.68 a |
| At anthesis (stage 2) | 0.75 de | 1.14 e | 0.90 f | 0.31 d | 1.07 d | 0.36 d | 1.01 c | 6.07 c | 1.08 d |
| At anthesis (stage 3) | 0.77 d | 1.46 d | 0.67 g | 0.36 b | 1.25 b | 0.42 b | 1.16 b | 6.56 b | 1.36 b |
| 10 DAA (stage 4) | 0.89 b | 1.44 d | 1.03 c | 0.18 g | 0.58 g | 0.19 g | 0.68 f | 3.66 d | 0.61 g |
| 20 DAA (stage 5) | 0.98 a | 1.69 c | 1.93 a | 0.21 f | 0.70 f | 0.24 f | 0.72 d | 3.06 e | 0.73 f |
| 30 DAA (stage 6) | 0.59 g | 1.77 b | 1.36 b | 0.23 e | 0.81 e | 0.25 e | 0.70 e | 2.76 f | 0.83 e |
| 30 DAA (stage 7) | 0.86 c | 2.00 a | 0.98 d | 0.34 c | 1.18 c | 0.39 c | 1.01 c | 1.82 g | 1.11 c |
z Refer to Table 1 for description of stage.
Table 3
Growth and flowering of ‘Beni no Mai’ Lilium ×elegans lily as influenced by bud removal dates and size of bulbils
| Treatment | Bulbil weight | Days to | No. of flowers | Plant height (cm) | No. of leaves | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Bud removal date | Bulbil size | Shoot emergence | Flowering | ||||
| 1 April | Large | 501 az | 209 ab | 293 b | 1.6 a | 50 ab | 45 a |
| Medium | 363 b | 211 ab | 294 ab | 1.3 ab | 47 ab | 44 a | |
| Small | 326 c | 210 ab | 296 ab | 1.3 ab | 48 ab | 42 b | |
| Extra small | 263 de | 211 ab | 297 ab | 1.0 b | 44 b | 34 bc | |
|
|
|||||||
| 13 April | Large | 348 b | 214 ab | 297 ab | 1.1 b | 50 ab | 42 b |
| Medium | 303 cd | 211 ab | 297 ab | 1.1 b | 49 ab | 40 b | |
| Small | 283 d | 211 ab | 296 ab | 1.0 b | 48 ab | 35 bc | |
| Extra small | 252 de | 214 a | 297 a | 1.0 b | 44 b | 30 c | |
|
|
|||||||
| 25 April | Large | 299 cd | 209 ab | 295 ab | 1.4 ab | 52 a | 42 b |
| Medium | 239 e | 212 ab | 297 ab | 1.2 ab | 46 b | 36 bc | |
| Small | 228 ef | 209 ab | 298 a | 1.3 ab | 47 ab | 37 bc | |
| Extra small | 197 f | 209 ab | 298 a | 1.0 b | 45 b | 30 c | |
|
|
|||||||
| No removal | Large | 292 cd | 209 ab | 298 ab | 1.4 ab | 50 ab | 42 b |
| Medium | 225 ef | 212 ab | 298 a | 1.3 ab | 49 ab | 35 bc | |
| Small | 221 ef | 212 ab | 299 a | 1.0 b | 44 b | 30 c | |
| Extra small | 198 f | 209 b | 298 a | 1.0 b | 44 b | 30 c | |
Table 4
Effect of sequential temperature treatment given to bulbils on the growth and flowering of ‘Beni no Mai’ Lilium × elegans lily
| Temp. (°C) treatment for 7 weeks (W) | Days to | No. of flowers | Plant height (cm) | No. of leaves | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 0 – 3 W (A) | 3 – 4 W (B) | 4 – 7 W (C) | Shoot emergence | Flowering | |||
| 2.5 | 10 | 2.5 | 207 abz | 304 ab | 2.0 abc | 64 bcde | 63 abcd |
| 2.5 | 10 | 5 | 207 ab | 303 abc | 2.0 abc | 65 bcd | 62 abcde |
| 2.5 | 10 | 7.5 | 206 abc | 303 abc | 2.0 abc | 63 cdeb | 61 abcde |
| 2.5 | 12.5 | 2.5 | 206 abc | 305 a | 2.0 abc | 61 efg | 58 bcdef |
| 2.5 | 12.5 | 5 | 205 abc | 301 abc | 1.8 c | 61 efg | 52 def |
| 2.5 | 12.5 | 7.5 | 205 abc | 303 abc | 2.0 abc | 65 bcd | 58 bcdef |
| 2.5 | 15 | 2.5 | 206 abc | 303 abc | 1.9 bc | 67 abcd | 57 bcdef |
| 2.5 | 15 | 5 | 206 abc | 303 abc | 2.0 abc | 67 abcd | 62 abcde |
| 2.5 | 15 | 7.5 | 206 abc | 303 abc | 1.9 abc | 71 ab | 65 abc |
|
|
|||||||
| 5 | 10 | 2.5 | 205 abc | 304 ab | 1.8 c | 64 bcde | 60 abcde |
| 5 | 10 | 5 | 205 abc | 301 abc | 2.0 abc | 64 bcde | 59 abcde |
| 5 | 10 | 7.5 | 207 ab | 302 abc | 2.0 abc | 69 abcd | 56 cdef |
| 5 | 12.5 | 2.5 | 208 a | 304 ab | 2.0 abc | 57 g | 49 f |
| 5 | 12.5 | 5 | 206 abc | 305 a | 2.0 abc | 58 fg | 51 ef |
| 5 | 12.5 | 7.5 | 206 abc | 303 abc | 2.0 abc | 57 g | 51 ef |
| 5 | 15 | 2.5 | 205 abc | 303 abc | 2.0 abc | 65 bcd | 56 cdef |
| 5 | 15 | 5 | 207ab | 303 abc | 2.0 abc | 62 de | 63 abcd |
| 5 | 15 | 7.5 | 206 abc | 302 abc | 1.9 bc | 61 de | 56 cdef |
|
|
|||||||
| 7.5 | 10 | 2.5 | 205 abc | 304 ab | 2.1 ab | 63 cde | 60 abcde |
| 7.5 | 10 | 5 | 207 ab | 302 abc | 2.0 abc | 63 cde | 60 abcde |
| 7.5 | 10 | 7.5 | 207 ab | 302 abc | 2.0 abc | 63 cde | 60 abcde |
| 7.5 | 12.5 | 2.5 | 203 bc | 300 bc | 2.0 abc | 65 bcd | 60 abcde |
| 7.5 | 12.5 | 5 | 203 bc | 299 d | 2.1 ab | 62 de | 62 abcde |
| 7.5 | 12.5 | 7.5 | 204 abc | 302 abc | 2.0 abc | 68 abcd | 70 a |
| 7.5 | 15 | 2.5 | 204 abc | 302 abc | 2.0 abc | 67 abcd | 62 abcde |
| 7.5 | 15 | 5 | 202 c | 300 bc | 2.2 a | 74 a | 68 ab |
| 7.5 | 15 | 7.5 | 203 bc | 301 abc | 2.0 abc | 70 abc | 63 abcd |
|
|
|||||||
| Level of significancey | |||||||
| Temp. (°C) at 0 – 3 W (A) | *** | *** | *** | *** | *** | ||
| Temp. (°C) at 3 – 4 W (B) | ** | ns | ns | *** | *** | ||
| Temp. (°C) at 4 – 7 W (C) | ns | *** | ns | * | ns | ||
| A × B | *** | *** | ns | *** | *** | ||
| A × C | ns | ns | ns | ns | ns | ||
| B × C | ns | ns | ns | ns | *** | ||
| A ×B × C | ns | * | * | *** | ns | ||
Table 5
Weight of bulbils, area of trapezium A, and T2 relaxation time corresponding to the peak position in relation to T2 relaxation time of 20 ms of ‘Beni no Mai’ Lilium ×elegans lily
| Harvesting of bulbils at number of days (D) after anthesis | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 0 D | 14 D | 28 D | 42 D | 56 D | 70 D | |
| Weight of bulbils (mg) | 138 az | 173 b | 284 c | 433 d | 632 e | 754 f |
| Area of trapeziumy | 0.22 a | 0.40 b | 0.44 bc | 0.56 d | 0.65 e | 0.83 f |
| Peak position of T2 relaxation time based on the T2 at 20 msy | −2.67, 17.33 | −1.13, 18.87 | −1.6, 18.83 | −0.44, 19.56 | +1.12, 21.12 | +3.16, 23.36 |
References
Faust, M., D. Liu, M.M. Millard, G.W. Stutte. 1991. Bound versus free water in dormant apple buds - A theory for endodormancy. HortScience. 26:887-890. https://doi.org/10.21273/HORTSCI.26.7.887

Gross, K.C. 1983. Changes in free galactose, myo-inositol and other monosaccharide in normal and non-ripening mutant tomatoes. Phytochemistry. 22:1137-1139. https://doi.org/10.1016/0031-9422(83)80207-6

Gross, K.C., S.J. Wallner. 1979. Degradation of cell wall polysaccharides during tomato fruit ripening. Plant Physiology. 63(1):117-120. https://doi.org/10.1104/pp.63.1.117



Gross, K.C., A.E. Watada, M.S. Kang, S.D. Kim, K.S. Kim, S.W. Lee. 1986. Biochemical changes associated with the ripening of hot pepper fruit. Physiologia Plantarum. 66:31-36. https://doi.org/10.1111/j.1399-3054.1986.tb01227.x

Horimoto, T., M. Koshioka, S. Kubota, L.N. Mander, N. Hirai, N. Ishida, J.K. Suh, A.K. Lee, M.S. Roh. 2011. Effect of warm and cold stratification on 1 H-NMR profiles, endogenous gibberellins and abscisic acid in Styrax japonicus seeds. Horticulture, Environment, and Biotechnology. 52:233-239.

Kano, H.N., T. Ishida, T. Kobayashi, M. Koizumi. 1990. 1H-NMR imaging analysis of changes in free water distribution in barley and soybean seeds during maturation. Japanese Journal of Crop Science. 59:503-509. https://doi.org/10.1626/jcs.59.503

Kim, K.J., C.H. Park, O.J. You, J.H. Shin. 1998. Effects of removing time of flower buds on root yield and paeoniflorin contemt in Paeonia lactiflora Pallas. Korean Journal of Medicinal Crop Science. 6(3):193-197.
Li, B.W., P.J. Schuhmann. 1980. Gas-liquid chromatographic analysis of sugars in ready-to-eat breakfast cereals. Journal of Food Science. 45(1):138-141. https://doi.org/10.1111/j.1365-2621.1980.tb03889.x

Liu, D., M. Faust, M.M. Millard, M.J. Line, G.W. Stutte. 1993. States of water in summer-dormant apple buds determined by proton magnetic resonance imaging. Journal of the American Society for Horticultural Science. 118(5):632-637. https://doi.org/10.21273/JASHS.118.5.632

Miao, Y., Z. Zhu, Q. Guo, H. Ma, Y. Yang, L. Zhu. 2014. Effects of flower bud removal and artificial pollination on growth and yield of Tulip edulis. China Journal of Chinese Materia Medica. 39(11):2016-1928.

Millard, M.M., D. Liu, M.J. Line, M. Faust. 1993. Method for imaging the states of water by nuclear magnetic resonance in low-water-containing apple bud and stem tissues. Journal of the American Society for Horticultural Science. 118(5):628-631. https://doi.org/10.21273/JASHS.118.5.628

Oglevee, J.R., J.F. Tammen, W. O’Donovan. 1986;Lily products. United States Patent No 4,604,824
Park, J.M., J.H. Kang, M.B. Kim. 2004. Growth and yield of Atractylodes japonica Koidz. affected by shading and flower bud pinching. Korean Journal of Medicinal Crop Science. 12(3):231-236.
Roberts, A.R., J.R. Stang, Y.T. Wang, W.R. McCorkle, L.J. Riddle, F.W. Moeller. 1985. Technical Bulletin 148 (pp. 74Agricultural Experiment Station, Oregon State University Corvallis. OR, USA:
Roh, M., J.A. Bentz, P. Wang, E. Li, M. Koshioka. 2004. Maturity and temperature stratification affect the germination of Styrax japonicus seeds. The Journal of Horticultural Science and Biotechnology. 79(4):645-651. https://doi.org/10.1080/14620316.2004.11511820

Roh, M.S. 1988. Leaf cutting propagation of Asiatic hybrid lily. North Am. Lily Soc. Yearbook. 87-91.
Roh, M.S. 1992;Method for producing Lilium elegans. United States Patent No 5,138,794
Roh, M.S. 1996. New production technology of Lilium - a review on propagation and forcing. Acta Horticulturae. 414:219-228. https://doi.org/10.17660/ActaHortic.1996.414.26

Roh, M.S. 2011. Controlled flowering in the genus Lilium - review of the past achievements and the future direction of research. Acta Horticulturae. 900:189-203. http://dx.doi.org/10.17660/ActaHortic.2011.900.23

Roh, M.S., R.J. Griesbach, K.C. Gross, M. Line. 1996. Identification and evaluation of the interspecific hybrid between Lilium longiflorum and L. callosum. . Acta Horticulturae. 414:111-124. https://doi.org/10.17660/ActaHortic.1996.414.11

Roh, M.S., A.K. Lee, J.K. Suh. 2007. Induction of bulb maturity of Ornithogalum thyrsoides. Scientia Horticulturae. 114:138-141. https://doi.org/10.1016/j.scienta.2007.06.004

Roh, S.M., H.F. Wilkins. 1977a. The effects of bulb vernalization and shoot photoperiod treatments on growth and flowering of Lilium longiflorum Thunb. cv. Nellie White. Journal of the American Society for Horticultural Science. 102:229-235. https://doi.org/10.21273/JASHS.102.3.229

Roh, S.M., H.F. Wilkins. 1977b. The physiology of dormancy and maturity of Lilium longiflorum Thunb. cv. Nellie White bulb. III. Maturity - Scale removal and bulb temperature treatment. Journal of the Korean Society for Horticultural Science. 18:187-193.
Roh, S.M., H.F. Wilkins. 1977c. The physiology of dormancy and maturity of Lilium longiflorum Thunb. cv, Nellie White bulb. IV. Dormancy and maturity - translocation in the double nose bulb. Journal of the Korean Society for Horticultural Science. 18:194-202.
Roh, S.M. 1978a. Dormancy and maturity in the bulbil of Lilium lancifolium. 1. The influence of low temperature, gibberellic acid, abscisic acid on the spouting response and endogenous growth substances in mature bulbil. Journal of the Korean Society Horticultural Science. 22:82-88.
Roh, S.M. 1978b. Dormancy and maturity in the bulbil of Lilium lancifolium 2. The influence of temperature treatment on the growth and flowering responses. Journal of the Korean Society Horticultural Science. 22:199-208.
Roh, S.M. 1982. Propagation of Lilium longiflorum Thunb. by leaf cutting. HortScience. 17:607-609.
Rose, J.K., K.A. Hadfield, J.M. Labavitch, A.B. Bennett. 1998. Temporal sequence of cell wall disassembly in rapidly ripening melon fruit. Plant Physiology. 117:345-361. https://doi.org/10.1104/pp.117.2.345



Rowland, L.J., D. Liu, M.M. Millard, M.J. Line. 1992. Magnetic resonance imaging of water in flower buds of blueberry. HortScience. 27:339-341. https://doi.org/10.21273/HORTSCI.27.4.339

Suh, J.K., M.S. Roh. 2014. New technique for cut flower production from bulbils of the Asiatic hybrid lily (Lilium × elegans Thunb.). Scientia Horticulturae. 165:374-383. https://doi.org/10.1016/j.scienta.2013.11.012

Wang, Y.T. 1981. The influence of air and soil temperatures on the growth and development of Easter lily, Lilium longiflorum, during different growth phases. MS Thesis. Oregon State University, Corvallis, OR, USA. 73pp.
Zhang, X., D.J. Beattie, J.W. White. 1990. Flower initiation in three hybrid lily cultivars. Acta Horticulturae. 266:183-188. https://doi.org/10.17660/ActaHortic.1990.266.23

- TOOLS







