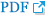|
 |
- Search
| J. People Plants Environ > Volume 23(4); 2020 > Article |
|
ABSTRACT
Background and objective: This study was conducted to examine changes in the composition and physiological activity of Gardenia Fructus after being roasted.
Methods: The antioxidant, anti-inflammatory and antibacterial activity of Gardenia Fructus was evaluated using the Gardenia Fructus (GF) and roasted Gardenia Fructus (RGF) ethanol extracts, and their components were analyzed through HPLC.
Results: As a result, it was confirmed that the content of gardenoside and geniposide decreased and the content of genipin increased when GF was roasted. The total content of polyphenols was 54.5 ┬▒ 2.18 mg gallic acid equivalents (GAE) per gram of the GF extract and 69.6 ┬▒ 0.36 mg GAE per gram of the RGF extract. As a result of evaluating 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, both the GF and RGF extracts showed the similar activity to ascorbic acid at the concentrations of 1 mg/mL or higher. In RAW264.7 macrophages stimulated by lipopolysaccharides (LPS), the RGF extract showed a higher effect of reducing NO production, and significantly reduced the expression of an inflammatory cytokine, IL-6. As a result of evaluating the antimicrobial activity, the RGF extract showed higher antimicrobial activity against Escherichia coli and Bacillus subtilis. In the dextran sulfate sodium salt (DSS) induced inflammatory bowel disease mouse model, the RGF extract reduced the weight of the spleen, and both the GF and RGF extracts reduced the number of bacteria in the colon.
Conclusion: Therefore, it has been confirmed through this study that roasting at a high temperature changes the main components of the GF extract and increases its biological activity. The RGF extract is expected to be used as a natural material with antioxidant, anti-inflammatory and antibacterial effects.
Methods: The antioxidant, anti-inflammatory and antibacterial activity of Gardenia Fructus was evaluated using the Gardenia Fructus (GF) and roasted Gardenia Fructus (RGF) ethanol extracts, and their components were analyzed through HPLC.
Results: As a result, it was confirmed that the content of gardenoside and geniposide decreased and the content of genipin increased when GF was roasted. The total content of polyphenols was 54.5 ┬▒ 2.18 mg gallic acid equivalents (GAE) per gram of the GF extract and 69.6 ┬▒ 0.36 mg GAE per gram of the RGF extract. As a result of evaluating 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, both the GF and RGF extracts showed the similar activity to ascorbic acid at the concentrations of 1 mg/mL or higher. In RAW264.7 macrophages stimulated by lipopolysaccharides (LPS), the RGF extract showed a higher effect of reducing NO production, and significantly reduced the expression of an inflammatory cytokine, IL-6. As a result of evaluating the antimicrobial activity, the RGF extract showed higher antimicrobial activity against Escherichia coli and Bacillus subtilis. In the dextran sulfate sodium salt (DSS) induced inflammatory bowel disease mouse model, the RGF extract reduced the weight of the spleen, and both the GF and RGF extracts reduced the number of bacteria in the colon.
Conclusion: Therefore, it has been confirmed through this study that roasting at a high temperature changes the main components of the GF extract and increases its biological activity. The RGF extract is expected to be used as a natural material with antioxidant, anti-inflammatory and antibacterial effects.
Gardenia Frustus is the dried fruits of Gardenia jasminoides Ellis which is, as a shrub of the family Rubiaceae and a tropical and subtropical plant, mostly planted in the southern region of Korea (Kang et al., 2011). Gardenia Frustus has been used for anti-inflammation, diuresis, hemostasis, diabetes, jaundice, gonorrhea, eye inflammation and hemoptysis (Kim et al., 2017).
Gardenia Frustus contains iridoid compounds such as genipin, geniposide and gardenoside, carotinoid compounds such as crocin and crocetin and flavonid compounds (Park et al., 2018). Genipin is an aglycone derived from geniposide which is hydrolyzed by intestinal bacteria (Yamazaki and Chiba, 2008). Geniposide is known to be effective in reducing triglyceride, phospholipid, malondialdehyde, glucose, GTP and free fatty acid (Yang et al., 2011), and genipin is known to inhibit nitric oxide (NO), having anti-inflammatory effects (Yamazaki and Chiba, 2008).
Natural products are used as they are without processing, or are used after being processed according to situations (Oh et al., 2009). Herbal medicine processing in oriental medicine is the pharmaceutic technique to alter the properties of raw materials and includes stir-frying, boiling, steaming and releasing, or other methods. The herb-processing is used to promote the efficacy of natural products or to reduce their toxicity and side effects (Nam et al., 2015). For example, Zizyphus jujuba Miller used as a tranquilizer is heated and roasted to increase its sedating effect by turning sanjoinine-A into sanjoinine-Ah1 (Lee et al., 2009), and heating was also reported to increase phenolic compounds within the body of plants (Park et al., 1993).
However, earlier studies on the efficacy of Gardenia Frustus mostly were mostly conducted without processing. Gardenia Frustus known to have various bioactive compounds is expected to show changes in its components and activity when it is roasted, but there has been no study on the effect of roasted Gardenia Frustus. Against this backdrop, this study aimed to assess the anti-inflammatory activity of the ethanol extract of Gardenia Frustus and roasted Gardenia Frustus using RAW 246.7 cells with inflammations induced by lipopolysaccharides (LPS) and an inflammatory bowel disease mouse model induced by dextran sulfate sodium salt (DSS) and to identify changes in the bioactivity of Gardenia Frustus depending on the heat treatment by measuring antioxidant and antimicrobial activity.
The Gardenia Frustus used in this study was supplied by the Department of Agronomy and Medicinal Plant Resources of Gyeongnam National University of Science and Technology. Roasted Gardenia Frustus was obtained by roasting Gardenia Frustus at 100 ┬░C for 5 minutes, and 1 kg of the Gardenia Frustus and roasted Gardenia Frustus powder respectively was extracted with 70% ethanol for 24 hours. The extracts were filtered and evaporated under reduced pressure, and were stored at 4 ┬░C. A total of 76.3 g of Gardenia Frustus extract and 70.8 g of roasted Gardenia Frustus extract were obtained.
As an experimental animal, 4-week male ICR mice were supplied by Orient (Korea). They were placed in a cage with a sterilized litter and the temperature and humidity of the cage were maintained at 20 ┬▒ 2 ┬░C and 50 ┬▒ 10% with the light/dark cycle of 12 hours. The mice were allowed to freely have feed and water. After the 1-week adaptation period, their weight was measured and they were randomly divided into six groups. This experiment was conducted with the approval of the Institutional Animal Care and Use Committee of Kunsan National University (Approval No. 2020-01).
DPPH radical scavenging activity was measured according to the method suggested by Senba et al. (1999) using 2,2-diphenyl-1-picrylhydrazyl (DPPH). On a 96-well plate, 100 ╬╝L of the extracts of different concentrations and 0.2 mM of DPPH were mixed and reacted at room temperature for 20 minutes, and their absorbance was measured at 517 nm. The group added with ethanol instead of the extracts was set as the negative control group, and the group added with L-ascorbic acid known to show high antioxidant activity was set as the positive control group. DPPH radical scavenging activity was calculated using the absorbance of the groups treated with the extracts and the non-treated groups.
The total content of polyphenols was measured according to the method suggested by Singleton and Rossi (1965) using Folin-Denis reagent. In this experiment, 200 ╬╝L of the Gardenia Frustus and roasted Gardenia Frustus extracts was mixed with 1 mL of 0.2 N Folin-Ciocalteu reagent and 800 ╬╝L of 7.5% Na2CO3 solution, and was reacted at room temperature for 2 hours with light blocked. After that, their absorbance was measured at 620 nm. Gallic acid was used as the standard reagent to obtain a standard curve and the content of polyphenols was calculated based on the curve. The total content of polyphenols was expressed as mg gallic acid equivalents (mg GAE) per gram of extract.
Mouse macrophages (RAW 264.7) were purchased from the Korean Cell Line Bank (KCLB 40071). The cells were cultured using a DulbeccoŌĆÖs modified eagle medium (DMEM) that contained 10% fetal bovine serum (FBS) and 1% penicillin/ streptomycin in an incubator (37 ┬░C, 5% CO2).
Cytotoxicity was measured using the crystal violet method (Equation 1; Ishiyama et al., 1996). RAW 264.7 cells were seeded in 24-well plates at a density of 2 ├Ś 105 cells per well, and were cultured in an incubator (37 ┬░C, 5% CO2) for 24 hours. After removing the media used to culture cells, the cells were treated with the Gardenia Frustus and roasted Gardenia Frustus extracts of different concentrations and were cultured for 24 hours additionally. After that, the media were removed, and were cleaned twice with 1X phosphate buffered saline (PBS). Cells were fixed for 10 minutes by adding 10 % formalin solution. After removing the formalin solution, the cells were dyed for 10 minutes by adding 1% crystal violet solution that was prepared using 20% methanol. The crystal violet solution was cleaned using distilled water and moisture was dried. The crystal violet dye in the cells was dissolved with 33% acetic acid, and the solution was moved into a 96-well plate to measure their absorbance at 595 nm.
The amount of nitric oxide (NO) within cells was measured with a kit using Griess reagent (KOMANITECH lnc., KOR). RAW 264.7 cells were seeded in 24-well plates at a density of 1 ├Ś 106 cells per well, and after pre-culture for 24 hours, the cells were treated with 1 ╬╝g/mL of lipopolysaccharides (LPS) and the Gardenia Frustus and roasted Gardenia Frustus extracts of different concentrations. The cells were cultured in an incubator (37 ┬░C, 5% CO2) for 24 hours, and the supernatant liquid was collected and reacted with the same amount of Griess reagent. Their absorbance was measured at 550 nm, and sodium nitrite was used as the standard reagent.
RAW 264.7 cells were seeded in 24-well plates at a density of 1 ├Ś 106 cells per well, and were pre-cultured for 24 hours. They were treated with 1 ╬╝g/mL of LPS and the Gardenia Frustus and roasted Gardenia Frustus extracts of different concentrations. The cells were cultured in an incubator (37 ┬░C, 5% CO2) for 24 hours, and the supernatant liquid was collected. The amount of inflammatory cytokine IL-6 was measured using an enzyme linked immunosorbent assay (ELISA) kit (AbFRONTIER, KOR).
RAW 264.7 cells were seeded in 24-well plates at a density of 1 ├Ś 106 cells per well and were fixed for 24 hours. After that, they were treated with 1 ╬╝g/mL of LPS and the Gardenia Frustus and roasted Gardenia Frustus extracts of different concentrations and were cultured for 24 hours. Using TRIzol reagent (Sigma Chemical, USA), their RNA was extracted, and the extracted RNA was quantified using QIAxpert (QIAGEN, DEU). Reverse transcription-polymerase chain reaction (RT-PCR) was performed using a RT-PCR premix kit (Bionner, Korea), The quantified total RNA and forward and reverse primers were added to make a reaction solution of 20 ╬╝L respectively. Electrophoresis was performed on the PCR products in the 1.2% agarose gel. The expression of genes in the control group was standardized using ╬▓-actin. The sequences of the primers used in this experiment were forward 5ŌĆ▓-TTCCATCCAGTTGCCTTCTT-3ŌĆ▓ and reverse 5ŌĆ▓-GGGAGTGGTATCCTCTGTGA-3ŌĆ▓.
Antimicrobial activity was measured using an ultrasensitive radial diffusion assay (URDA; Lehrer et al., 1991). The Gardenia Frustus and roasted Gardenia Frustus extracts were produced using 0.01% acetic acid (HAc), and the strains used in this experiment include Bacillus subtilis KCTC1021 (Gram-positive), Escherichia coli D31 (Gram-negative) and Candida albicans (mycotic). Each strain was inoculated into a tryptic soy broth (TSB) and was activated at 37 ┬░C for 24 hours. After that, their absorbance was measured at 630 nm to ensure the O.D value of each bacterium was 0.01 (ŌēÆ 0.5 McF). To the TSB of 9.5 mL containing 0.03% TSB, 1% agarose and 10 mMp hosphate buffer, 500 ╬╝L of the prepared bacterial solution was poured in the plate to solidify it. On the solidified plate, wells (diameter: 2.5 mm) were bored and 5 ╬╝L of the Gardenia Frustus and roasted Gardenia Frustus extracts was injected into each well to ensure they are absorbed into the plate. For the next 3 hours, they were cultured at 37 ┬░C primarily, and after that, 10 mL of the overlay medium containing 6% TSB, 1% agarose and 10 mMph osphate buffer was poured to solidify it. They were cultured at 37 ┬░C for 24 hours secondarily, and the size of clear zones created in the wells was measured to assess antimicrobial activity. As the negative control group, 0.01% HAc was used.
Experimental animals were divided into the normal, control, DSS+Gardenia Frustus extract (C-GF), DSS+roasted Gardenia Frustus extract (C-RGF), Gardenia Frustus extract (GF) and roasted Gardenia Frustus extract (RGF) groups, and each group had six animals. The Gardenia Frustus and roasted Gardenia Frustus extracts were prepared using distilled water and their concentration was set to be 100 mg/kg. For 10 days, 100 ╬╝L of the extracts was orally administered at the same time everyday. Groups other than the normal and extract-treated groups were ensured to freely have 2.5% DSS dissolved in distilled water from the 4th day of administering the extracts in order to induce an inflammatory bowel disease, and the normal and extract-treated groups had water freely. The weight of animals was measured at the same time everyday during the period of this experiment, and symptoms such as diarrhea and bloody stools were observed.
On the 7th day after the administration of DSS, mice were sacrificed, and their colon and spleen were removed. To identify the enlarged spleen that is observed in systemic inflammatory response syndrome, the weight of the spleen was measured and its relative weight was determined by expressing it as a percentage (%) against the weight of mice. The length from the appendix to the anus was measured, and stools and some tissue were collected from the removed colon and were added with 1X PBS sterilized 4 times more than the weight of each. They were homogenized using a homogenizer and the supernatant liquid was collected and was diluted 108 times. After that, they were smeared by 100 ╬╝L in solid media, and were cultured at 37 ┬░C for 48 hours. The number of colonies were counted to identify bacterial translocation.
Gardenia Frustus and roasted Gardenia Frustus extracts were analyzed using high performance liquid chromatography (HPLC). The HPLC used in this experiment was Agilent 1260 series (Agilent Technologi, USA), and Luna C18 columns (250 ├Ś 4.6 mm, 5 ╬╝m), a reverse-type column, were used. The Gardenia Frustus and roasted Gardenia Frustus extracts were produced using methanol, and their concentration was 1 mg/mL. The produced extracts were filtered using a 0.45 ╬╝m syringe filter. The mobile phases consisted of methanol (A) and 0.1% formic acid in water (B), and as a gradient elution system HPLC was performed under the following conditions: 0ŌĆō4 minutes 90% B; and 4ŌĆō40 minutes 10% B. The flow rate was 0.5 mL/min, and the amount of the extracts injected was 20 ╬╝L. The results of analysis were observed at 240 nm.
To assess the antioxidant activity of the Gardenia Fructus (GF) and roasted Gardenia Fructus (RGF) extracts, DPPH radical scavenging activity was measured, and the scavenging activity at the concentration of 1 and 2 mg/mL was 85.1 ┬▒ 0.58% and 95.9 ┬▒ 1.94% in the GF group, and 84.1 ┬▒ 0.99% and 90.6 ┬▒ 1.51% in the RGF group respectively, showing a concentration-dependent increase. At the concentration of 2 mg/mL, the GF and RGF groups showed higher radical scavenging activity than the positive control group, L-ascorbic acid (90.2 ┬▒ 0.26%; Fig. 1).
The total content of polyphenols in the GF and RGF groups was measured to be 54.5 ┬▒ 2.18 mg GAE/g and 69.6 ┬▒ 0.36 mg GAE/g respectively, showing a higher content in the RGF group (Table 1). The content of polyphenols and DPPH radical scavenging activity were reported to have a significant correlation in several studies, but no close correlation was found in this study.
The antimicrobial activity of the Gardenia Frustus (GF) and roasted Gardenia Frustus (RGF) extracts was assessed using B. subtilis, C. albicans and E. coli D31, and both the GF and RGF groups showed antimicrobial activity against E. coli D31 and B. subtilis. In the case of B. subtilis, the GF and RGF groups showed a clear zone the size of 7.17 mm and 7.01 mm respectively at the concentration of 500 mg/mL (Table 2). In the case of E. coli D31, no microbial activity was observed in the GF group at the concentration of 100 mg/mL, but the RGF group showed a slight trace of bacterial inhibition near the border of wells. At the concentration of 500 mg/mL, the GF and RGF groups showed a clear zone the size of 7.51 mm and 9.35 mm respectively, showing a higher antibacterial activity. In the case of mycotic C. albicans, both the GF and RGF groups did not show any bacterial inhibition (Fig. 2).
To examine the effects of the Gardenia Frustus (GF) and roasted Gardenia Frustus (RGF) extracts on RAW 264.7 cells, a crystal violet assay was performed. As a result, the GF and RGF groups showed a concentration-dependent decrease in cell viability. At the concentration of 1 mg/mL, the GF and RGF groups showed the cell viability of 98.1 ┬▒ 2.75% and 100 ┬▒ 0.71% respectively, showing no cytotoxicity, but at the concentration of 2.5 mg/mL, the groups showed the cell viability of 74.7 ┬▒ 2.30% and 70.6 ┬▒ 2.91%, showing low cytotoxicity. At the concentration of 10 mg/mL, the GF and RGF groups showed the cell viability of 17.8 ┬▒ 2.58% and 25.5 ┬▒ 4.62% respectively, showing nearly 80 % of cytotoxicity (Fig. 3).
Nitric oxide (NO) produced by iNOS plays an important role as a neurotransmitter in the nervous and immune systems and remove bacteria or tumors, but the excessive production acts as a key factor in inflammatory reactions, inducing inflammations (Djeraba et al., 2002).
Changes in the amount of NO production in LPS-induced RAW 264.7 cells depending on the concentration of the GF and RGF extracts were measured, and the amount of NO production was inhibited concentration-dependently. The GF and RGF extracts at the concentration of 1 mg/mL inhibited NO production to the level of 88.5 % and 69.0 % of LPS respectively, and at the concentration of 10 mg/mL, it was inhibited to the level of 18.0% and 8.9% respectively, which showed the RGF extract has a higher inhibition effect (Fig. 4).
IL-6 is known to react to infections or damage to tissues in the immune system and is produced by stem cells including T cells, mononuclear phagocytes and fibroblasts and non-stem cells, inducing acute inflammatory reactions (Feghali and Wright, 1997; Rana et al., 2013).
The amount of IL-6 production in LPS-induced RAW 264.7 cells depending on the concentration of the GF and RGF extracts, and the GF and RGF extracts start to significantly inhibit the production of IL-6 increased by LPS from the concentration of 2.5 mg/mL. At the highest concentration (10 mg/mL), the GF and RGF extracts inhibited the production of IL-6 to the level of 7.81% and 5.80% of the LPS-treated group respectively. To verify that these results were related to the expression of the IL-6 gene. RT-PCR was conducted, and the GF and RGF extracts were found to reduce the expression of IL-6 increased by LPS concentration-dependently, showing a higher inhibiting effect in the RGF extract (Fig. 5).
The colon of mice was removed and the length from the appendix to the anus was measured. Compared to the normal group, the length in all the treated groups showed a decrease but the difference was not statistically significant. The length in the C-GF group administered with the Gardenia Frustus extract was 9.98 ┬▒ 1.11 cm, showing the highest decrease, and that in the C-RGF and RGF groups administered with the roasted Gardenia Frustus extract showed the similar length to the normal group.
To observe the spleen enlarged by inflammations, the weight of the removed spleen was measured and its relative weight was determined by expressing it as a percentage (%) against the weight of mice. Compared to the normal group, no enlargement caused by inflammations was observed in the control group, but the C-GF and C-RGF groups treated with the extracts showed a decrease in the weight of the spleen compared to the control group. The weight of the spleen in the C-RGF group was 0.24 ┬▒ 0.03%, showing the lowest weight. Given these results, the GF and RGF extracts did not affect the restoration of the length of the colon but were effective in reducing the weight of the spleen, and RGF extract was found to be more effective (Fig. 6).
The number of bacteria in the stools and some tissue collected from the isolated colon was counted, and the number of intestinal bacteria in the stools and tissue was found to be increased by the administration of DSS. The C-GF and C-RGF groups injected with DSS as well as the Gardenia Frustus and roasted Gardenia Frustus extracts showed a statistically significant decrease in the number of bacteria increased by DSS, and a higher decrease in the number of bacteria was observed in the stools in the group treated with the GF extract and the tissue in the group treated with the RGF extract. Given these results, the GF and RGF extracts were effective in reducing the number of intestinal bacteria increased by inflammations, and are expected to reduce the symptoms of inflammatory bowel diseases (Fig. 7).
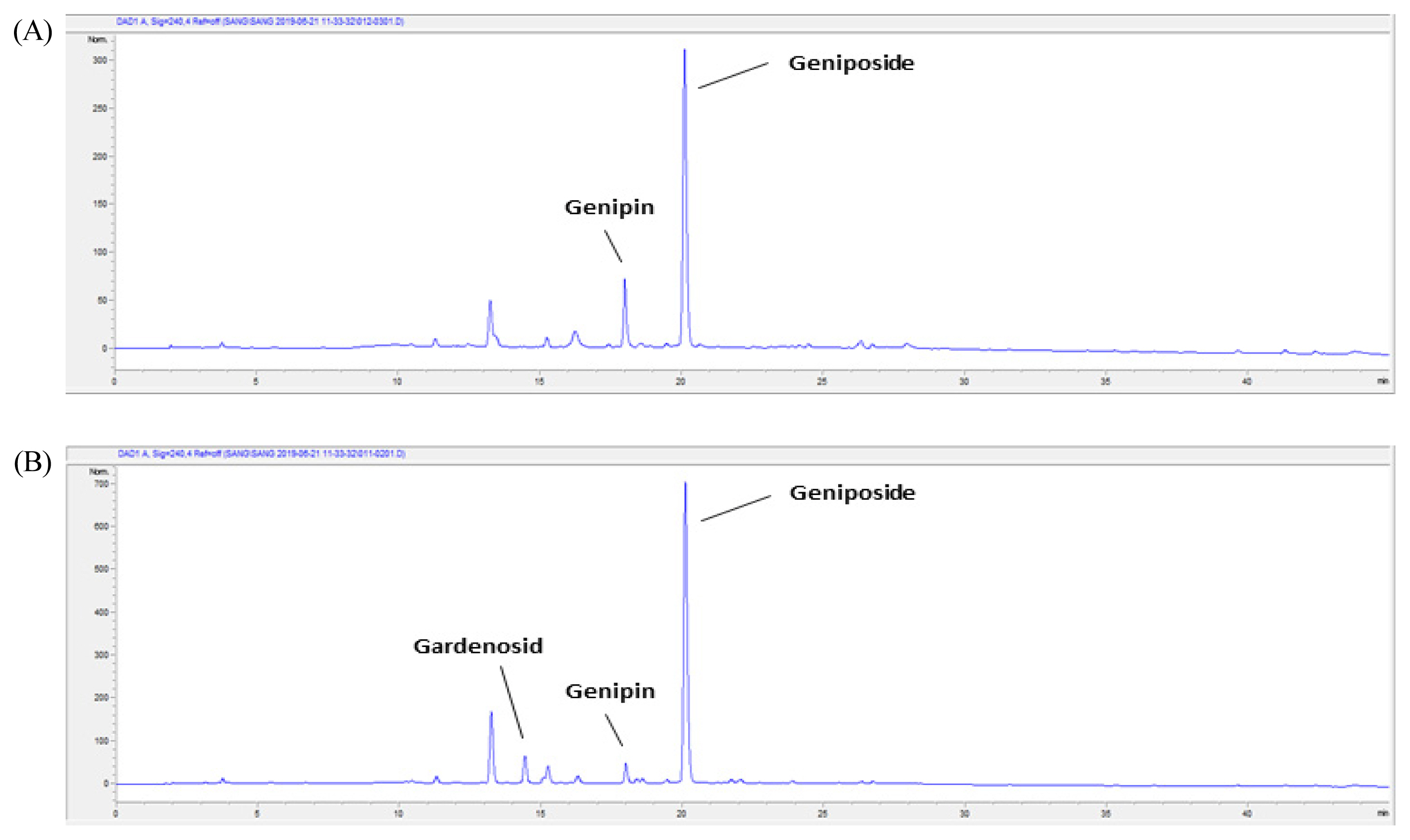
The Gardenia Frustus (GF) and roasted Gardenia Frustus (RGF) extracts were analyzed using high performance liquid chromatography (HPLC), and the peak of gardenoside, genipin and geniposide, key components, was observed at the minute of 14.4, 18.0 and 20.1 respectively (Table 3). The GF and RGF extracts contained geniposide the most, and the RGF extract showed a decrease in the content of geniposide. The content of genipin in the RGF extract was higher than the GF extract, and gardenoside was detected only in the GF extract. Therefore, roasting seemed to reduce the content of gardenoside and geniposide in Gardenia Frustus, and to convert some geniposide into genipin with sugar removed, increasing the content of genipin (Fig. 8).
This study examined the effects of Gardenia Frustus roasted at a high temperature on changes in antioxidant, antimicrobial and anti-inflammatory activity using the Gardenia Frustus and roasted Gardenia Frustus ethanol extracts. The Gardenia Frustus and roasted Gardenia Frustus extracts were analyzed using HPLC, and roasting was found to reduce the content of gardenoside and geniposide in Gardenia Frustus and increase the content of genipin. Some geniposide seemed to be converted into genipin, and gardenoside and geniposide seemed to be converted into essential oil components (Park et al., 2011). The antioxidant activity of the Gardenia Frustus and roasted Gardenia Frustus extracts was assessed, and both of the extracts showed an increase in DPPH radical scavenging activity concentration-dependently, and at the concentration of 1 mg/mL or higher, their antioxidant activity was similar to that of ascorbic acid, a typical antioxidant substance. The total content of polyphenols in the roasted Gardenia Frustus extract was higher than the Gardenia Frustus extract, which coincides with the result that roasting increased the content of phenolic compounds in plants (Park et al., 1993). The antimicrobial activity of the Gardenia Frustus and roasted Gardenia Frustus extracts was also assessed, and roasting increased the antimicrobial activity against E. coli D31. The amount of nitric oxide (NO) and IL-6 expression in LPS-induced RAW 264.7 cells were measured, and the roasted Gardenia Frustus extract showed a higher inhibiting effect, which can be attributed to the increasing content of genipin that has anti-inflammatory effects such as inhibiting the production of NO (Park et al., 1993). In the inflammatory bowel disease mouse model induced by dextran sulfate sodium salt (DSS) used in this study, the roasted Gardenia Frustus reduced the enlargement of the spleen caused by inflammations and the number of intestinal bacteria increased by DSS. Given these results, roasting seemed to be an effective method in changing the components of Gardenia Frustus and thus increasing bioactivity, and both Gardenia Frustus and roasted Gardenia Frustus can be utilized in various fields as a natural material with antioxidant, anti-inflammatory and antimicrobial activity.
Acknowledgements
This research was supported by funds of National Research Foundation (NRF-2019R1A4A1026423).
Fig.┬Ā1
DPPH radical scavenging activity at various concentrations of GF (Gardenia Fructus) and RGF (roasted Gardenia Fructus) extracts. Error bars represent standard deviation of data set. Asterisks indicate significance compared to control (*p < .05, **p < .01, ***p < .001).
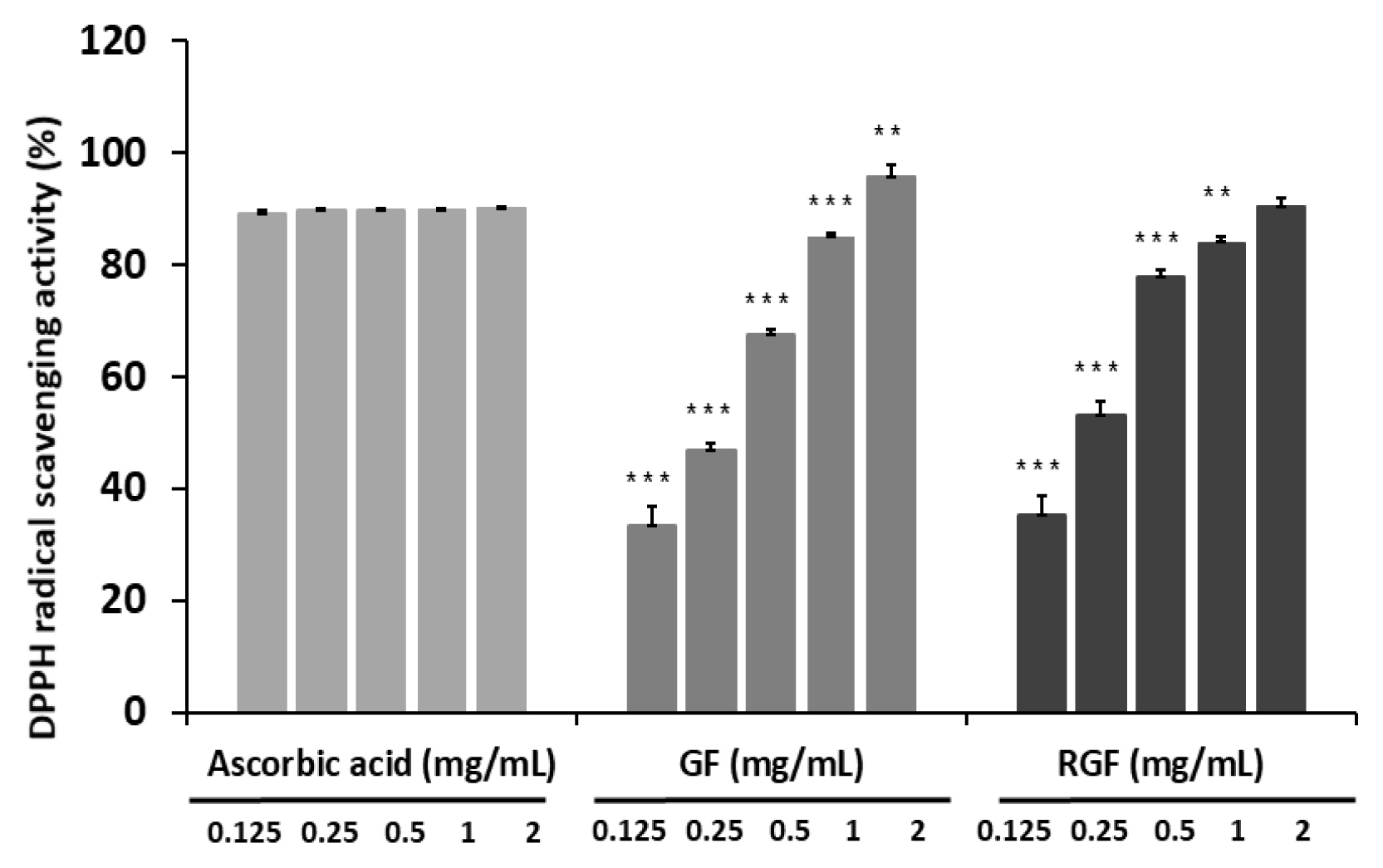
Fig.┬Ā2
Antimicrobial activities of GF (Gardenia Fructus) and RGF (roasted Gardenia Fructus) extracts against Bacillus subtilis, Candida albicans and Escherichia coli D31.
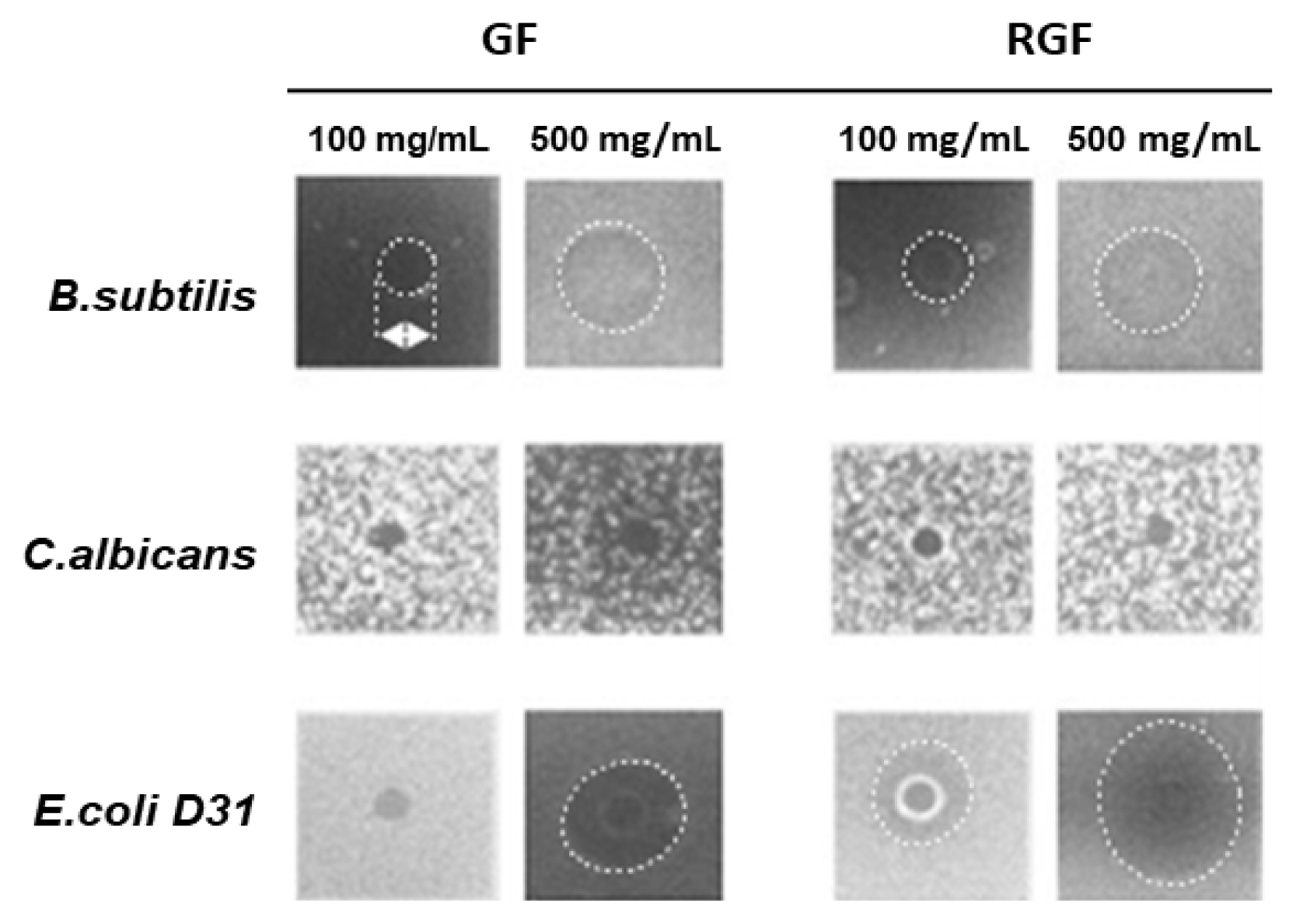
Fig.┬Ā3
Effects of GF (Gardenia Fructus) and RGF (roasted Gardenia Fructus) extracts on RAW264.7 cell viability. Cells were treated with different concentration of sample and incubated for 24 hr. Error bars represent standard deviation of data set. Asterisks indicate significance compared to control (*p < .05, **p < .01, ***p < .001).
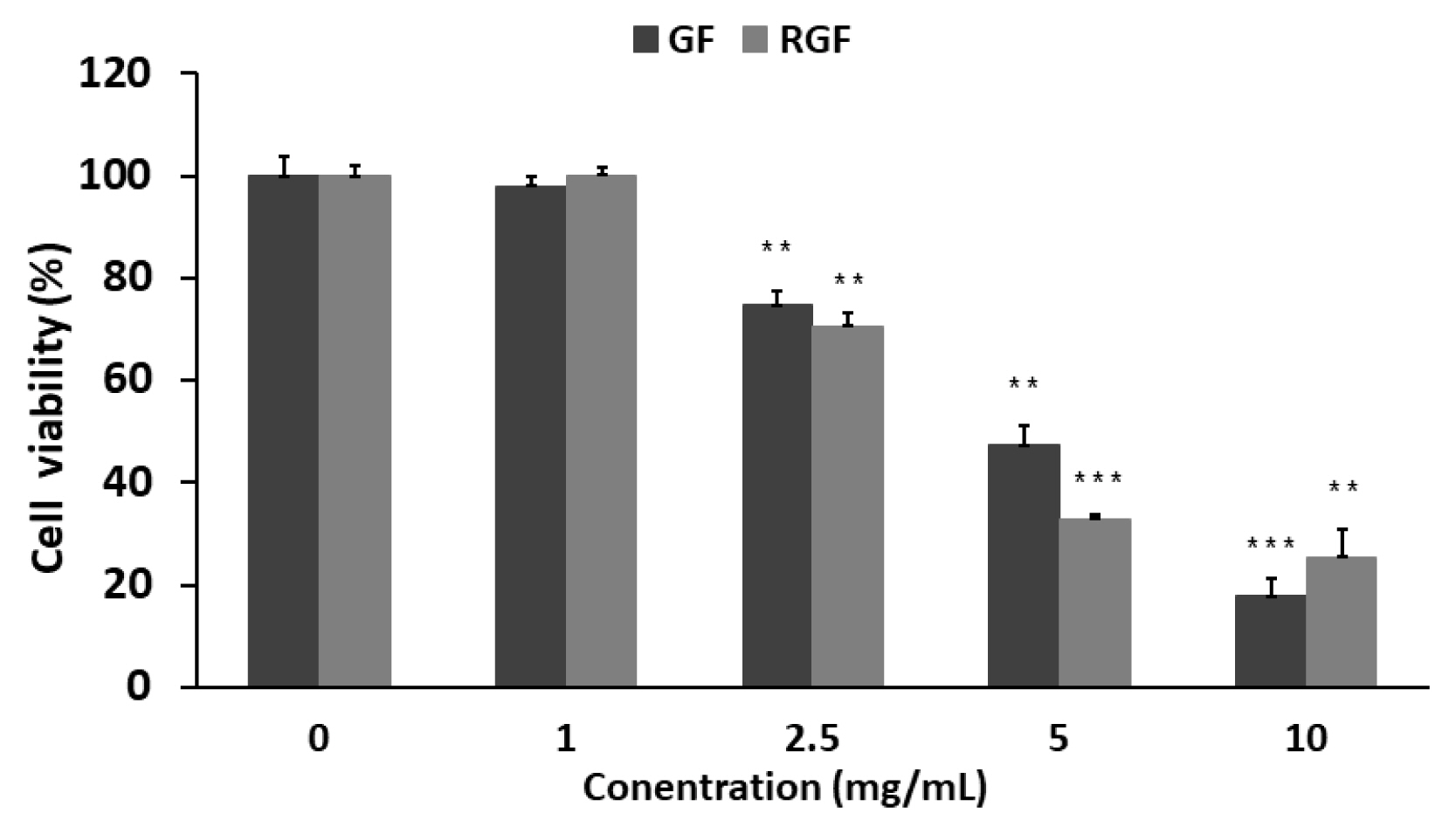
Fig.┬Ā4
Effects of GF (Gardenia Fructus) and RGF (roasted Gardenia Fructus) extracts on nitric oxide production in LPS-stimulated RAW264.7 cells. NO production was measured in the medium of RAW264.7 cells treated with different concentration of sample and LPS (1 ╬╝g/mL), and incubated for 24 hr. Error bars represent standard deviation of data set. Asterisks indicate significance compared to control (*p < .05, **p < .01, ***p < .001).

Fig.┬Ā5
Effects of GF (Gardenia Fructus) and RGF (roasted Gardenia Fructus) extracts on IL-6 expression in LPS-stimulated RAW 264.7 cells. Cells were treated with different concentration of sample and LPS (1 ╬╝g/mL), and incubated for 24 hr. (A) The production of IL-6 in cultured medium was measured using ELISA. Error bars represent standard deviation of data set. Asterisks indicate significance compared to LPS treated group (*p < .05, **p < .01, ***p < .001). (B) The mRNA level of IL-6 was determined using RT-PCR.

Fig.┬Ā6
Effect of GF (Gardenia Fructus) and RGF (roasted Gardenia Fructus) extracts on the changes in colon length (A) and relative spleen weight (B) of mice with colitis. Experimental colitis was induced by 2.5% DSS drinking water for seven days in mice. Error bars represent standard deviation of data set. Asterisks indicate significance compared to control (*p < .05).

Fig.┬Ā7
Effect of GF (Gardenia Fructus) and RGF (roasted Gardenia Fructus) extracts on stool colony (A) and colon colony (B) of mice with colitis. Experimental colitis was induced by 2.5% DSS drinking water for seven days in mice. Error bars represent standard deviation of data set. Asterisks indicate significance compared to control (*p < .05, **p < .01, ***p < .001).
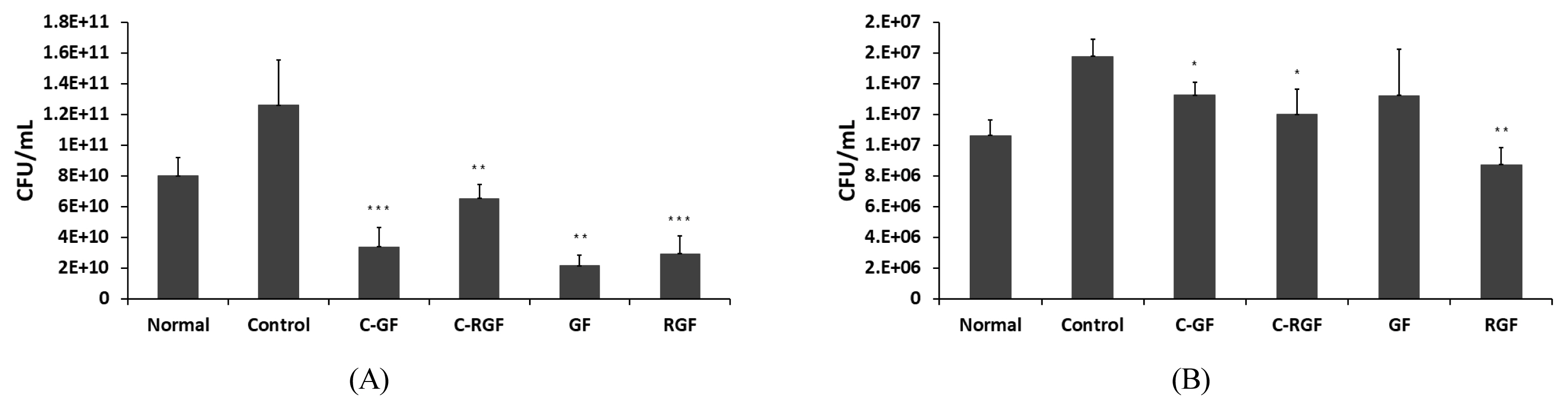
Fig.┬Ā8
HPLC chromatogram at 240 nm of GF (Gardenia Fructus; A) and RGF (roasted Gardenia Fructus; B) extracts.
Table┬Ā1
Total polyphenol contents of GF (Gardenia Fructus) and RGF (roasted Gardenia Fructus) extracts
| Extracts | Total polyphenol concentrations (mg GAE/g) |
|---|---|
| GF | 54.5 ┬▒ 2.18 |
| RGF | 69.6 ┬▒ 0.36 |
Table┬Ā2
Size of clear zone by GF (Gardenia Fructus) and RGF (roasted Gardenia Fructus) extracts
Table┬Ā3
Compounds identified in the GF (Gardenia Fructus) and RGF (roasted Gardenia Fructus) extracts by HPLC
References
Djeraba, A, E Musset, N Bernardet, Y Le Vern, P Qu├®r├®. 2002. Similar pattern of iNOS expression, NO production and cytokine response in genetic and vaccination-acquired resistance to MarekŌĆÖs disease. Vet Immunol Immunopathol. 85(1ŌĆō2):63-75.
https://doi.org/10.1016/s0165-2427(01)00412-3


Feghali, CA, TM Wright. 1997. Cytokines in acute and chronic inflammation. Front Biosci. 2:d12-26.
https://doi.org/10.2741/a171


Ishiyama, M, H Tominaga, M Shiga, K Sasamoto, Y Ohkura, K Ueno. 1996. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull. 19(11):1518-1520.
https://doi.org/10.1248/bpb.19.1518


Kang, SR, EY Park, MS Park, JH Park, YC Kim. 2011. Antioxidative and collagen synthetic abilities of Gardeniae Fructus and Saururus chinensis water extracts. J Invest Cosmetol. 7(2):165-171.
https://doi.org/10.15810/jic.2011.7.2.010

Kim, KH, SM Kim, SH Shin, YJ Lee, WS Baek. 2017. Quality monitoring of specification standard of Gardeniae fructus in the Korean pharmacopoeia and studies HPLC standard chromatogram. Korea J Herbol. 32(2):97-105.
https://doi.org/10.6116/kjh.2017.32.2.97

Lee, EK, HG Hong, MS Chong. 2009. Study on the comparison of effects by extraction methods of roast and raw Semen Zizyphi Spinosae. Korean J Orient Physiol Pathol. 23(6):1416-1422.
Lehrer, RI, M Rosenman, SS Harwig, R Jackson, P Eisenhauer. 1991. Ultrasensitive assays for endogenous antimicrobial polypeptides. J Immunol Methods. 137(2):167-173.
https://doi.org/10.1016/0022-1759(91)90021-7


Nam, HH, HJ Kim, NJ Choi, SS Roh, BK Choo. 2015. A comparison of antioxidant activity from Schisandra chinensis water extracts depending on stir-frying and stir-frying with liquids process. Korean J Org Agric. 23(4):987-997.

Oh, JH, JS Sin, ES Ahn, SJ Lee, JC Lee, JH Lim, SK Hong, JK Hong, YJ Lee. 2009. A literature survey of the modern techniques used for the processing of herbal medicines. Korean J Pharm Sci. 39(4):275-297.
https://doi.org/10.4333/KPS.2009.39.4.275

Park, DS, SJ Kim, SH Jeong, IB Seo. 2011. Effects of Gyeonbi-tang treatment on the monosodium iodoacetate-induced mild osteoarthritis in rats. Korean J Orient Physiol Pathol. 25(1):84-91.
Park, MH, KC Kim, JS Kim. 1993. Changes in the physicochemical properties of ginseng by roasting. J Ginseng Res. 17(3):228-231.
Park, MK, HJ Yoon, HJ Lee. 2018. Antioxidant effect and inhibitory activities of ethyl acetate fraction from Gardenia jasminoides extract on nitric oxide production and pancreatic cancer cell proliferation. Korean J Food Sci Technol. 50(2):209-215.
https://doi.org/10.9721/KJFST.2018.50.2.209

Rana, SV, S Sharama, SK Sinha, KK Parasad, A Malik, K Singh. 2013. Pro-inflammatory and anti-inflammatory cytokine response in diarrhoea-predominant irritable bowel syndrome patients. Trop Gastroenterol. 33(4):251-256.
https://doi.org10.7869/tg.2012.66

Senba, Y, T nishishita, K Saito, H Yoshioka, H Yoshioka. 1999. Stopped-flow and spectrophotometric study on radical scavenging by tea catechins and the model compounds. Chem Pharm Bull. 47(10):1369-1374.
https://doi.org/10.1248/cpb.47.1369

Singleton, VL, JA Rossi. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 16(3):144-158.

Yamazaki, M, K Chiba. 2008. Genipin exhibits neurotrophic effects through a common signaling pathway in nitric oxide synthase-expressing cells. Eur J Pharmacol. 581(3):255-261.
https://doi.org/10.1016/j.ejphar.2007.12.001


Yang, HJ, MJ Park, HS Lee. 2011. Antioxidative activities and components of Gardenia jasminoides
. Korean J Food Sci Technol. 43(1):51-57.
https://doi.org/10.9721/KJFST.2011.43.1.051