Introduction
Man-made chemicals/naturally occurring chemicals are used to protect the health of plants, animals, and humans as well as to add economic or aesthetic value to plants and animals for their products. In agriculture, these chemicals are applied directly to crops almost at all stages of growth from planting to harvest storage or transport. Besides, the soil may be treated pre-planting and during plant growth for control of weeds and other pests which are abundant in agricultural soils. There are numerous chemicals for agricultural use in many ways of delivering to the target with concentration and formulation varying to suit the mode of application and the target. Pesticides are commonly applied and are used worldwide in agriculture to protect crops from pests and in public health to control diseases that are transmitted by vectors (Ramakrishnan et al., 2010).
Accidental spills/leaks occurring during transport and storage of industrial materials & agricultural chemicals have polluted areas that were never intended as sites for waste disposal. Thus, soil and water bodies serve as ultimate sink/reservoir for all kinds of pesticides whether they are aimed intentionally/unintentionally applied. The interaction between pesticides and non-target organisms present in soil/water bodies eventually leads to not only alteration in life-function of individual organisms but also disturbance in equilibrium among organisms thereby affecting the primary life support function of natural resources - soil and water bodies (Pimental and Levitan, 1986; Ramakrishnan et al., 2010). Impact of organic pollutants, in particular, agrochemicals on the capacity of aquatic/soil environments to support life has been the subject of extensive research worldwide as documented in earlier reviews (Ramakrishnan et al., 2010; Venkateswarlu, 1993). The influence of agrochemicals including organophosphates on non-target organisms is dependent on their concentrations prevailing in the environment after their entry.
Understanding of biotic and abiotic factors on the fate of organophosphorus pesticides in the environment is essential. Among organophosphates, quinalphos is one of the major insecticides used in agriculture.
Quinalphos and its metabolite - 2-HQ toxicity
Quinalphos (O, O-diethyl O-quinoxaline-2-yl phosphorothioate), is one of the widely used organophosphorus broad-band based pesticide with both insecticidal and acaricidal properties. World Health Organization (WHO, 2004) assessed all agrochemicals used in the globe and ranked quinalphos is moderately hazardous. Because of its nature, it is either banned or restricted for usage in most of the nations. Quinalphos which is classified as a yellow label (highly toxic) pesticide in India, is widely used in large and repeated doses to control insect pests that attack many crops in agriculture and horticulture fields. Quinalphos shows high-level efficacy against a wide range of chewing, sucking, biting and leaf-mining pests, particularly in the orders of Lepidoptera, Homoptera, Coleoptera, Diptera, and Thysanoptera (Meyes, 1985). Because of its effective control of all pests over different crops, only 1% of the pesticide applied, hits the target pest, while the remaining 99% of the pesticide drifts into the environment contaminating soil, water and biota (Pimental and Levitan, 1986; Ramakrishnan et al., 2010) and disrupting the biogeochemical cycling in nature.
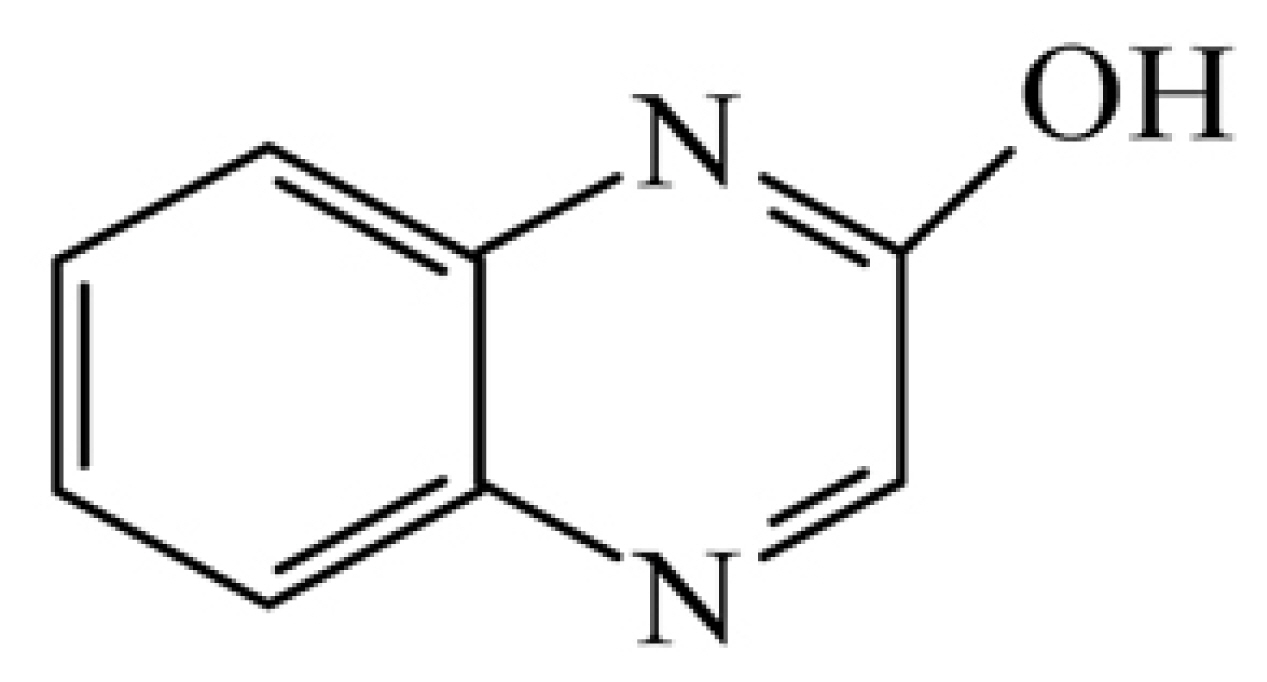
Despite extensive usage of quinalphos in agriculture for control of pests over crops, inhibition of acetylcholinesterase (AChE) was reported in non-target organisms by quinalphos (Chebbi and David, 2009; Kopecka et al., 2004; Pan and Dutta, 1998; Silman and Sussman, 2005; Vig et al., 2006). Quinalphos adversely influence on blood and brain esterase activity in chickens (Vairamuthu and Thanikachalam, 2003), fish fertility efficiency in adult male rats (Sarkar et al., 2000) and also genotoxicity to silver barb (Sadiqul et al., 2017). A 3-year (2000ŌĆō2002) evaluation report (Teixeira et al., 2004) of forensic pesticide and herbicide intoxications in Portugal indicated about 29% of total cases due to quinalphos. Primary toxicity due to acetylcholinesterase inhibition is terminated by the hydrolysis of quinalphos and its metabolic products containing phosphorous detected in human serum and urine (Vasili─ć et al., 1992). There was the least attention focused on biotic factors than on abiotic factors in fate of quinalphos in natural resources (Babu et al., 1998; Banerjee and Dureja, 1999; Gangireddygari, Kalva, et al., 2017; Gon├¦alves et al., 2006; Gupta et al., 2011; Kaur and Sud, 2012; Subba Reddy, 2013; Talwar et al., 2014). Despite the complete disappearance of quinalphos from soils within two weeks, its hydrolytic metabolite, 2-hydroxyquinoxaline was accumulated (Babu et al., 1998). As the secondary toxicity of the metabolite, 2-hydroxyquinoxaline (Fig. 1) equally poses a severe concern as the primary toxicity of the parent compound - quinalphos from the point of environmental safety. The presence of metabolites in soils may also exert their interactions with microflora and their microbial activities, thereby affecting soil fertility. The impact of metaboliteson microbial activities and biochemical transformations in soil received less attention in comparison to other parental insecticide metabolites. Knowledge of biotic factors involved in the fate of metabolite - 2-hydroxyquinoxaline is validated.
The structural resemblance of 2-HQ - Quinoline and its degradation by microorganisms
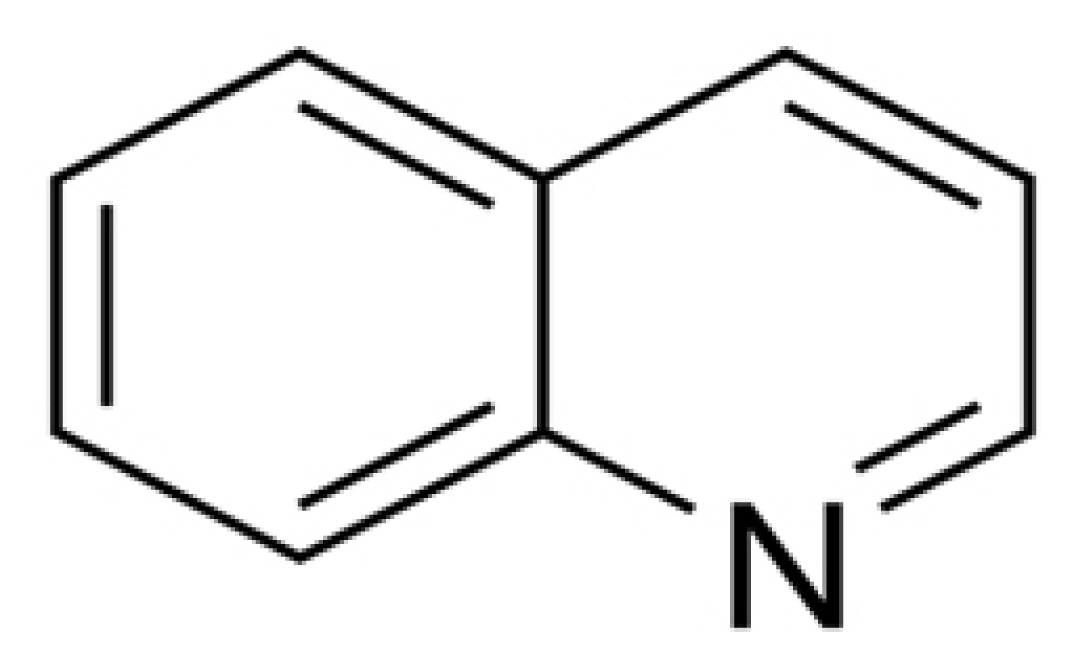
Quinoline (C9H7N; Fig. 2) isan aromatic compound formed when benzene ring fuses with the pyridine ring, is an N-heterocyclic compound, occurs naturally in the environment and is used in numerous industrial processes. 2-Hydroxyquinoxaline (2-HQ; C8H6N2O) is an aromatic and bicyclic compound with the fusion of one benzene ring and one pyrazine ring and is getting accumulated in the environment-soil due to its slow degradation upon repeated applications of the parent compound-quinalphos (Babu et al., 1998). Quinoline is distinct from 2-HQ (2 nd position is occupied by a hydroxy group and additional nitrogen in the fourth position of the compound) also detected in the environment due to the production and application of quinoline-based drugs, quinoline-based dyes and petroleum products (Environmental Protection Agency, 2001; Fetzner, 1998; Padoley et al., 2008; Tuo et al., 2012). Good solubility, high mobility, and persistence of quinoline cause that it is detected in both aquatic and soil ecosystems (Blum et al., 2011; Hartnik et al., 2007; Reineke et al., 2007). Ecotoxicological effect of quinoline has been demonstrated toward bacteria, algae, daphnids, and soil invertebrates (Kobeti─Źov├Ī et al., 2011; Sochov├Ī et al., 2011). It is also, genotoxic and mutagenic activities (Eisentraeger et al., 2008; Neuwoehner et al., 2009).
It is desirable to have microbial/bacterial cultures in our stockpile for mitigation of N-heterocyclic compounds associated toxicity by enhancing the degradation of N-heterocyclic compounds in the environment. The number of bacterial cultures such as Bacillus sp. (Tuo et al., 2012), Pseudomonas sp. (Bai et al., 2009; Lin and Jianlong, 2010; Sun et al., 2009), Burkholderia pickettii (Jianlong et al., 2002) and Rhodococcus sp. (Zhu et al., 2008), Comamonas sp. (Cui et al., 2004); Brevundimonas sp. K4 (Wang et al., 2014); Pseudomonas citronellosis PY1 (Wang et al., 2020) and so on with the capacity to tolerate and degrade quinoline were reported.
In addition to reports of bacterial culture, there were few reports on the degradation of quinoline by fungal species. White rot fungi possess an ability to degrade pharmaceuticals, pesticides, and dyes (Cruz-Morató et al., 2013). Species of Aspergillus, Mucor, or Cochliobolus are widely used in the elimination of dangerous xenobiotics (Carvalho et al., 2011; Felczak et al., 2014; Krupiński et al., 2014). Also, fungi belonging to the genus of Cunninghamella are well known for their ability to metabolize various pollutants. Cunninghamella species transform polyaromatic hydrocarbons like phenanthrene (Marco-Urrea et al., 2015; Pothuluri et al., 1996), tributyltin compounds (Bernat and Dlugoński, 2002), or drugs (Ahmad et al., 2014; Paludo et al., 2013). C. elegans IM1785/21Gp removes quinoline with high efficiency and transforms the pollutant into two novel hydroxylated derivatives, 2-hydroxyquinoline and 3-hydroxyquinoline (Felczak et al., 2016).
There were so many reports on the complete degradation and removal of quinoline by different microbial cultures, produced different types of metabolites (Bl├żse et al., 1996; Fetzner, 1998; Lin and Jianlong et al., 2010; OŌĆÖLoughlin et al., 1996; Pereira et al., 1988; Schwarz et al., 1988; Sun et al., 2009) ultimately one common metabolite of molecular formula (C9H6O3) having 8-hydroxy coumarin and then finally converted into atmospheric carbon dioxide and ammonium nitrogen (Wang et al., 2020). Whereas in the case of 2-hydroxyquinoxaline there is no much information on the complete degradation of the compound.
Abiotic and biotic hydrolysis of quinalphos
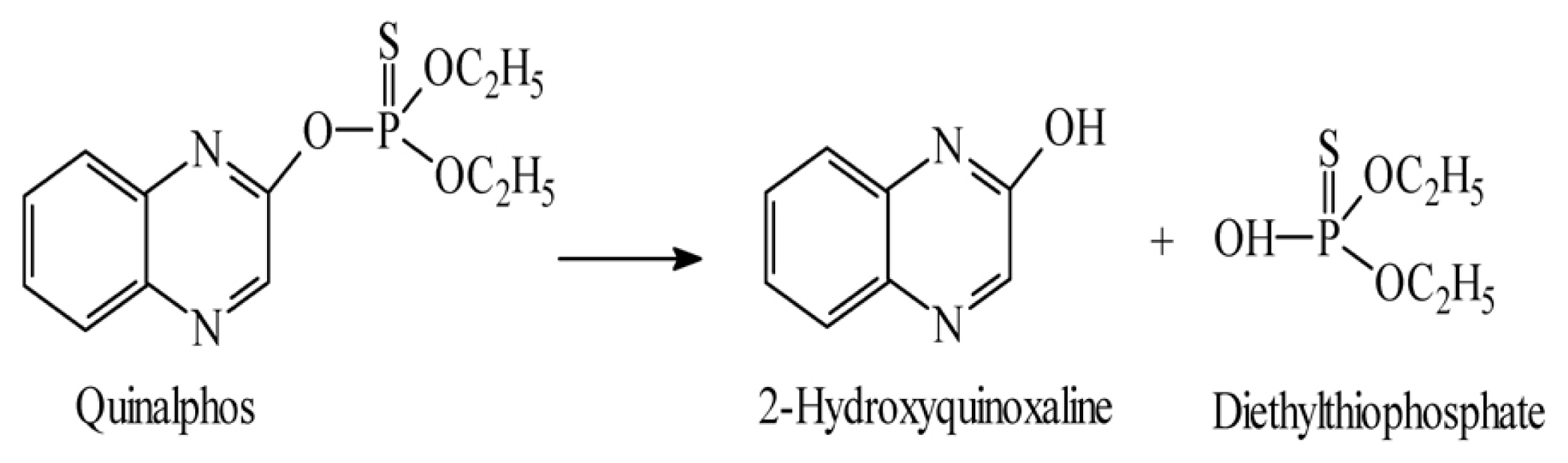
Hydrolysis of the ester bond to the aromatic moiety in quinalphos leads to the formation of 2-hydroxyquinoxaline which has been identified as the main metabolite in soil, in water, and on crops (Awasti and Ahuja, 1989; Babu et al., 1998; Menon and Gopal, 2003). The 2-Hydroxyquinoxaline metabolite was formed through pH-independent hydrolysis probably mediated by microbes, proceeding with a further breakdown (Menon and Gopal, 2003). In the same study hydrolysis of quinalphos followed two pathways depending on the nature of the cation. 2-Hydroxyquinoxaline (nucleophilic attack at the aliphatic C) was formed in the presence of Na, K, and Ca-clays. Esbata et al. studied the degradation of quinalphos under controlled conditions in the basic aqueous solutions in the presence and absence of MnO2 and TiO2 with pH range 11.8ŌĆō13.6 at five different temperatures 25, 30, 35, 40, and 45┬░C by following spectrophotometrically the appearance of the product 2-hydroxyquinoxaline (Esbata et al., 2017; Fig. 3).
Dureja et al. (1988) also studied the photodegradation of quinalphos in distilled, tap, and rainwater and in aqueous acetone by exposure to natural sunlight formed 2-hydroxyquinoxaline. In another study about the photolytic degradation of quinalphos in natural waters and on soil matrices under simulated solar irradiation conditions reported 2-hydroxyquinoxaline is the main metabolite (Gon├¦alves et al., 2006). Decay profile and metabolism of quinalphos were studied in soil, water, and plants reported 2-hydroxyquinoxaline is the main metabolite and more toxic than the parent compound and persist for a long time (Gupta et al., 2011). A combination of TiO2 and UV light together caused more rapid degradation of quinalphos and formed to quinalphos-oxon, hydroxylated quinalphos and dealkylation of phosphate moiety (Kaur and Sud, 2012). An isolated bacterial strain - Ochrobactrum sp. strain HZM degraded quinalphos and produced 2-hydroxyquinoxaline as the main metabolite (Talwar et al., 2014). Gangireddygari, Kanderi, et al. (2017) also reported that quinalphos was degraded by Bacillus subtilis and formed 2-hydroxyquinoxaline as the main metabolite (Fig. 3).
Effects of 2-HQ on organisms
This study had been initiated by observations in quinalphos exposed mammals and birds, which exhibited an obvious impairment of immunological parameters along with a decrease in melatonin (Haldar, unpublished data). Since melatonin easily scavenges free radicals and several other oxidants, it can be consumed by reactive intermediates and a decline as observed may indicate oxidative stress (Hardeland et al., 1999; Hardeland and Coto-Montes, 2000; Hardeland et al., 2000). Concerning melatonin immunomodulatory properties (Guerrero and Reiter, 2002; Haldar, 2002; Hardeland et al., 2005), a drop in melatonin level would be also under immunological dysfunctions. Since the organophosphate quinalphos does not dispose of reactive groups capable of inducing oxidative stress directly, we assumed secondary toxicity induced by the metabolite 2-HQ an electron-rich molecule containing two nitrogen atoms and carrying a hydroxyl group, which should easily undergo redox reactions, perhaps via an intermediate quinoxaline-2-oxyl radical. In vitro, 2-HQ can destroy vitamin C and E, oxidizing various biogenic amines such as serotonin, melatonin, the melatonin metabolite - N1-acetyl-5-methoxykynuramine (AMK), dopamine, norepinephrine, epinephrine, and unsubstituted and substituted anthranilic acid derivatives when exposed to light (Behrends et al., 2004; Behrends et al., 2007).
2-HQ toxicity was studied in several phylogenetically unrelated organisms such as rotifer - Philodina acuticornis, dinoflagellate - Lingulodinium polyedrum and ciliate - Paramecium bursaria and proved it induces oxidative stress and mutations. Philodinais an established model organism for testing oxidotoxicity, antioxidant effects and gerontoprotection (Meadow and Burrows, 1971; Poeggler and Hardeland, 2001; Poeggeler et al., 2005). 2-HQ treated Philodina acuticornis body size is dropped and the life span is also reduced. In the dinoflagellate, 2-HQ strongly induce the oxidative stress by strongly increasing protein carbonyl, a major oxidative protein modification. In an entirely different test organism, the ciliate Paramecium bursaria, which is also sensitive to oxidotoxins, 2-HQ caused an inhibition of cell proliferation and at the higher concentration decreased cell number.
In an Ames test of mutagenicity using the particularly suitable and sensitive, histidine-auxotroph strain Salmonella typhimurium TA-102, 2-HQ proved to be genotoxic for both light and dark exposed bacteria (Riediger et al., 2007).
Influence of 2-HQ on the bacterial growth rate
To find out the growth kinetics of 2-HQ on a bacterium, minimal salts medium (MSM) fortified with 2-HQ at a concentration of 50 ppm mLŌłÆ1 in 250 mL of Erlenmeyer flasks with the two bacterial species - Bacillus sp. and Ochrobactrum sp. HQ1 isolated from soil. (Subba Reddy et al., 2014; Subba Reddy et al., 2016). The flasks were incubated in an orbital shaker at 175 rpm at 37┬░C for 24 hr. Five-milliliter aliquots from growing culture broth were withdrawn aseptically at 6 hr intervals and growth monitored at 600 nm in a UV-Visible spectrophotometer (Chemito UV-2600). The total number of viable bacterial colony-forming units was determined by the serial dilution method on nutrient agar medium. The specific growth rate of two bacterial isolates Bacillus sp. and Ochrobactrumsp. HQ1 was calculated in the logarithmic phase as per the equation - K= logNt-log N0/log 2 ├Ś t.
The viable cell count in the growing culture of bacteria on 2-HQ was measured at regular intervals of 6 hrs. At the beginning (0 times) immediately after inoculation the viable cell count of is 28├Ś109 and 84├Ś109 CFU/mL was recorded in respect of Bacillus sp. and Ochrobacturm sp. HQ1. After incubation for 6 hr, the initial total viable cell count rose to 50├Ś109 and 158├Ś109 CFU/mL in the culture of Bacillus sp. and Ochrobacturmsp. HQ1. During this log phase both the bacterial species, growth rate and generation time calculated. Doubling time of Bacillus sp. and Ochrobacturm sp. HQ1 at the log/exponential phase is 0.79 h or 47.4 and 0.71 h and 42.6 min/generation, respectively (Subba Reddy et al., 2014; Subba Reddy et al., 2016).
To date, there is no detailed research report on the effect of 2-hydroxyquinoxaline on species-specific soil micro-organism comparing with other bacterial and fungal species with other agrochemicals available in the market.
Effect of 2-HQ on soil enzyme activities
Soil is a living, dynamic and nonrenewable source. The total sustainability of soilnatural functions depends on soil quality and health and the delicate balance among different microorganisms that determine the recycling of nitrogen, carbon and other valuable plant nutrients. The entry of agro-chemicals including pesticides into the soil due to agricultural practices can influence equilibrium among microflora in the soil, thereby affecting soil fertility. Soil natural functions under the influence of agrochemicals continuously need to be monitored using key indicators of biological and biochemical parameters. Because of rapid formation of 2-hydroxyquinoxaline from a widely used organophosphorus insecticide, quinalphos in soil, the impact of 2-hydroxyquinoxaline at two concentrations 2 and 10 ppm on nitrogen mineralization and six important soil enzyme (Cellulase and amylase, protease, urease, phosphatase, and dehydrogenase) activities representative of carbon, nitrogen, and phosphorus cycles were assessed in two soils - black soil [black soil properties -sand (%)-74, silt (%)- 16, clay (%)-10, texture-loamy sand, alkaline nature, pH-8.1; Electrical conductivity (ds/m)2 - 0.048, organic matter (%) - 1.34, water holding capacity - 40, available nutrients (Kg/ha) - N-200. P2O5-15.4, K2O5 -183]; and red soil [red soil properties - sand (%)-70 silt (%)- 23 clay (%)-07 - sandy loam and, acidic, pH-6.4, Electrical conductivity (ds/m)2- 0.230, organic matter (%) - 0.536, water holding capacity - 23, available nutrients (Kg/ha) - N-226. P2O5-56.4, K2O5-149] collected from groundnut field for a laboratory study (Narahari Kumar, 2005).
Effect of 2-HQ on nitrogen mineralization
Mineralization of exogenously added organic peptone in soils treated with 2- hydroxyquinoxaline was studied aerobic conditions. The total ammonifying activity of soils is the sum of different forms of inorganic nitrogen (NH4 + - N + NO2 ŌłÆ - N + NO3 ŌłÆ- N). The application of 2-hydroxyquinoline was not toxic to ammonification in red soil even at a higher concentration of 10 ppm. But, ammonifying activity was depressed in 2-hydroxyquinoxaline amended black soil at both concentrations on the 10th day of incubation in comparison to control. This inhibition was alleviated in the black soil at a later stage of incubation. Quantification of nitrite and nitrate, formed due to oxidation of ammonium in soil, represents total nitrifying activity. Like ammonification, nitrification was not influenced by the presence of 2-hydroxyquinoxaline at both concentrations in the red soil. But, 2-hydroxyquinoxaline was stimulatory to nitrification in the black soil (Narahari Kumar, 2005)
Effect of 2-HQ on cellulase and amylase activity
2-Hdroxyquinoxaline was toxic to cellulase activity in the black soil at both concentrations on earlier interval 10th day of incubation. About 19 and 37% decreases in cellulase activity occurred in 2-hydroxyquinoxaline-amended black soil at 2 and 10 ppm, respectively on the 10th day of incubation. Recovery of cellulase activity from this incubation was made at the later stage of incubation. 2-hydroxyquinoxaline was less toxic to cellulase activity in the red soil. 2-Hydroxyquinoxaline was innocuous to amylase activity in both soils even at a higher concentration of 10 ppm (Narahari Kumar, 2005).
Effect of 2-HQ on protease activity
Protease activity was tested in black and red soils of groundnut fields with the concentrations of 2 and 10 ppm, 2-Hydroxyquinoxaline was not toxic to protease activity in red soil at both concentrations 2 and 10 ppm. But the same metabolite enhanced protease activity in the black soil on the 10th day of incubations at both concentrations (Narahari Kumar, 2005).
Effect of 2-HQ on urease activity
To determine the urease activity in both black and red soil, soil samples were fortified with 2-hydroxyquinoxaline at concentrations of 2 and 10 ppm. Application of 2-hydroxyquinoxaline even at higher concentration 10 ppm did not affect urease activity in both soils (Narahari Kumar, 2005).
Effect of 2-HQ on phosphatase activity
Phosphatase activity was measured in black and red soils of groundnut fields with the concentrations of 2 and 10 ppm. The occurrence of the rate of hydrolysis of p-nitrophenyl phosphate to the same extent in both soils with/without 2-hydroxyquinoxaline indicating the innocuous nature of the metabolite. Nearly sixty microorganisms of p-nitrophenol per g of soil were released from p-nitrophenyl phosphate in control and 2-hydroxyquinoxaline-amended black soil on the 10th day of incubation (Narahari Kumar, 2005).
Effect of 2-HQ on dehydrogenase activity
Narahari Kumar (2005), demonstrated the dehydrogenase activity in red and black soils. Soil samples were spiked with 2-hydroxyquinoxaline at concentrations of 2 and 10 ppm and enzyme activity were measured. 2-Hydroxyquinoxaline reduced dehydrogenase activity in the black soil at higher concentrations. The same metabolite at a lower concentration, 2 ppm was innocuous to dehydrogenase activity in the black soil. Depression in dehydrogenase activity in the red soil amended with 2-hydroxyquinoxaline even at low concentration, 2 ppm was recorded (Narahari Kumar, 2005).
2-HQ degradation in minimal salts medium
There was the least attention on the degradation of 2-HQ, comparison to degradation studies of other organophosphates - chlorpyrifos, methyl parathion, and its metabolite-p-nitrophenol and other metabolites in the environment. 2-Hydroxyquinoxaline degrading bacterial species were isolated from soil samples by selective enrichment method (Subba Reddy et al., 2014: Subba Reddy et al., 2016). This is the first research report on the isolation, identification, and characterization of microorganisms capable to degrade 2-HQ as the sole source of carbon and energy in minimal salts medium. A recent report evidenced that, the 2-HQ degradation was recorded by a novel aerobic gram-positive bacterium species - Bacillussp. and aerobic gram-negative bacterium - Ochrobactrum sp. HQ1 was identified (Subba Reddy et al., 2014: Subba Reddy et al., 2016). The authors were tested degradation efficiency of bacterial cultures by the addition of additional sources (0.01%) of different types of carbon (glucose and sodium acetate) and nitrogen sources (ammonium chloride, ammonium sulfate, urea and yeast extract). Among the additional carbon and nitrogen sources, carbon sources did not influence the degradation rate of 2-HQ by both species, but nitrogen sources - yeast extract marginally enhanced the rate of degradation of 2-HQ by both bacterial species - Bacillus sp. and Ochrobactrumsp. HQ1 (Subba Reddy et al., 2014: Subba Reddy et al., 2016). In another way effect of biotic factors tested, such as the size of inoculum (0.5 OD, 0.75 OD, and 1.0 OD) and co-culturing studies on the degradation of 2-HQ, but 1.0 OD of inoculum significantly improved the degradation rate of 2-hydroxyquinoxaline compared to lower OD inoculum size. Co-culturing conditions of 2-HQ degradation were evaluated with two bacterial species, results unmasked that, there was no significant improvement in the rate of degradation of 2-HQ in a mixed culture of Ochrobactrum sp. HQ1 and Bacillus bacterium HQ2 when compared with the individual bacterial cultures degradation (Subba Reddy, 2013). The optimal conditions for 2-HQ degradation by both potential bacterial cultures - Bacillus sp. and Ochrobactrum sp. HQ1, pH of 7ŌĆō8, the temperature of 37ŌĆō45┬░C and 2-HQ concentration of 500 ppm (Subba Reddy et al., 2014; Subba Reddy et al., 2016).
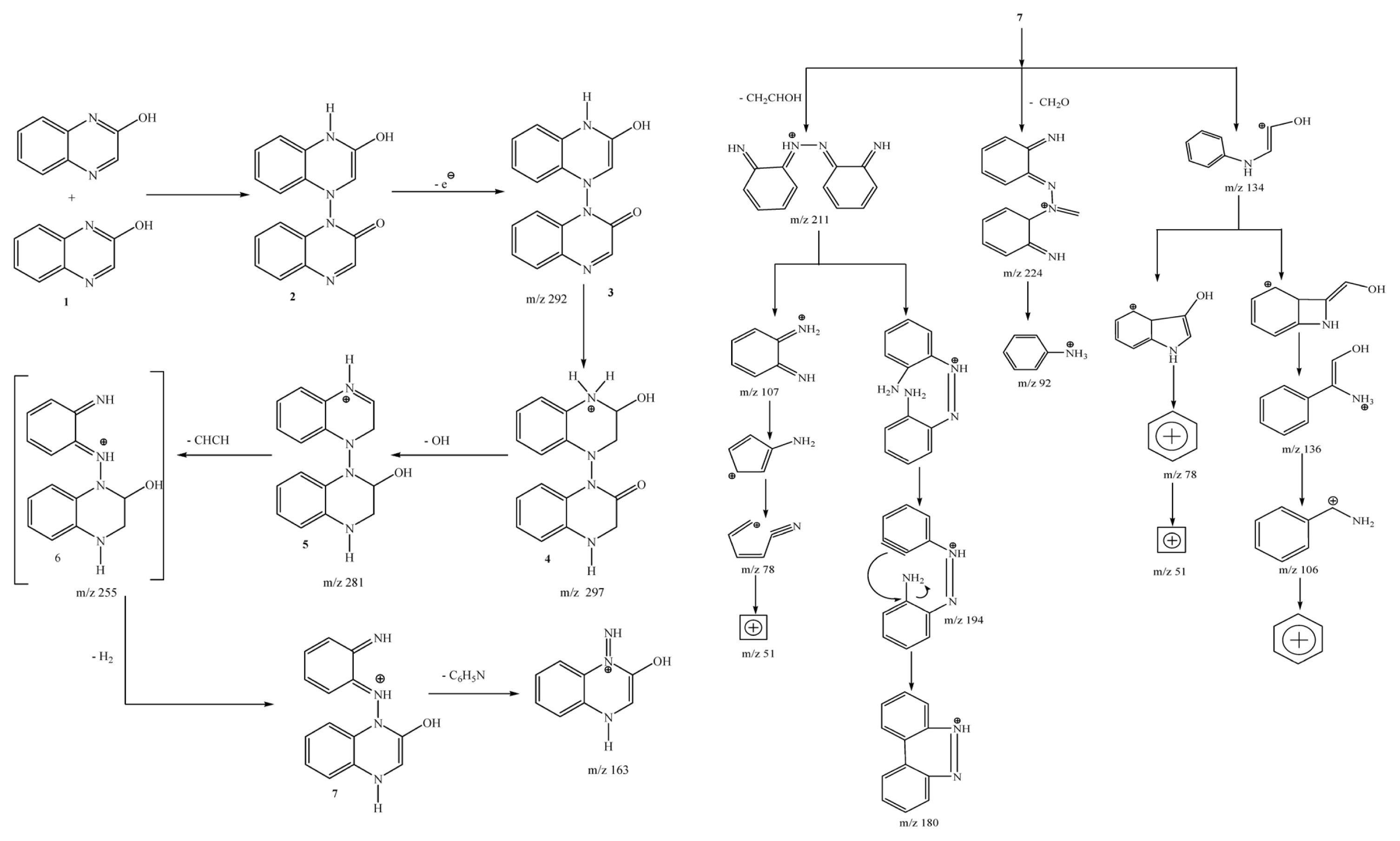
GC-MS analysis report evidenced that, the tentative 2-HQ degradation pathway has been elucidated with the bacterial species of Bacillus sp. (Subba Reddy et al., 2014; Fig. 4) and Ochrobactrum sp. HQ1 (Subba Reddy et al., 2016). In that degradation pathways initially, two molecules of 2-hydroxyquinoxaline molecules joined together to form a dimer with a molecular mass of 292 and proceed with the degradation process. Among the various metabolites m/z values of the GC-MS chromatogram, one of the metabolites with m/z value of 207 would be expected with the opening of pyrazine ring in dimer metabolite (Subba Reddy et al., 2014; Subba Reddy et al., 2016). Two types of bacterial species show same pattern on degradation of 2-HQ but variation in their fragmentation and intermediate molecules. The pyrazine nucleus possesses remarkable pharmaceutical importance and biological activities, and some of their derivatives occur as natural products.
Conclusion
The primary toxicity of quinalphos is due to acetylcholinesterase action, on the hydrolysis of quinalphos, which gives 2-HQ, which shown to be mutagenic, carcinogenic, genotoxic in organisms and intoxication of humans too. Secondary toxicity of 2-HQ was assessed in a limited manner. So, it needs to be further studied in-depth on how 2-HQ interacts with other soil biota and vertebrates. In red soil, 2-HQ was innocuous to ammonification, nitrification, cellulase, amylase, protease, urease, and phosphatase activity at both concentrations of 2 and 10 ppm but it was only toxic to dehydrogenase activity at 2 and 10 ppm. But whereas in black soil, 2-HQ was innocuous to nitrification, amylase, protease, urease, and phosphatase but depressed and toxic effect on ammonification and cellulase activity even at low and high concentrations of 2 and 10 ppm respectively. All these enzymes are involved in the recycling of carbon, nitrogen and phosphorus in the environment. During the degradation of 2-HQ by soil microbes, different types of metabolite are going to be formed. These metabolites are heterocyclic organic moieties. Due to the in-nocuousactivity of soil enzymes on 2-HQ, its metabolites may be mineralization by soil enzymes and play a significant role in the recycling of carbon, nitrogen and phosphorus in the environment.