 |
 |
- Search
| J. People Plants Environ > Volume 22(6); 2019 > Article |
|
ABSTRACT
Moss is used as an important material in indoor landscaping as well as outdoor landscaping. Moss is vivid green during growth and excellent in ornamental value. But when temperature drops, moss stops growth, turns brown or loses its ornamental value. In the present experiment, for the purpose of classifying native mosses according to the growth response to low temperature, the temperature of the plant growth chamber was set to 15┬░C/5┬░C (16h/8h, day/night) and 5┬░C (24h) for 8 weeks using nine native moss species. Thereafter, the temperature of the plant growth chamber was set to 20┬░C, and then the changes of moss block area and moss color were measured. The changes of moss block area and moss color were measured using a Photoshop program, after each moss block was photographed. As a result, Atrichum undulatum (Hedw.). Beauv., Etodon luridus (Griff.) A. Jaeger, Bachythecium plumosum (Hedw.) Schimp, Plagiomnium cuspidatum (Hedw.) T.J. Kop, and Hypnum plumaeforme Wilson showed a small decrease in moss block area at low temperature, and their recovery were the fastest at 20┬░C. These three species had higher green values at low temperature compared to other species, and the greenness increased rapidly at 20┬░C. On the other hand, Atrichum undulatum (Hedw.). Beauv., Marchantia polymorpha L., and Thuidium cymbifolium (Mitt.) A. Jaeger showed the smallest block area at low temperature and the lowest recovery even at 20┬░C. Their green values also decreased significantly at low temperature, and maintained low green value even at 20┬░C. These results showed that these three moss species are sensitive to low temperature. The remaining Myuroclada maximowiczii, Plagiomnium cuspidatum, and H. erectiusculum showed moderate responses to low temperature compared to other six species of mosses.
By botanical classification, moss is somewhere between chlorophyte and pteridophyte, divided into mosses (musci) and liverworts (hepaticae), with approximately 14,000ŌĆō16,000 species (National Institute of Biological Resources, 2015). Moss is comprised of stems, leaves and rhizoids, and some types have the form of cormus differentiated from stem and leaf, and some in which the leaves and stems are not differentiated like Marchantia and Anthocerotophyta (Choi, 1980). They have roots but most are comprised of rhizoids, without vascular bundle developed (Choi, 1980). Thus, most bryophytes absorb moisture through the entire plant body (During, 1992). The gametophyte generation is the plant body of moss we commonly see, living in the form of cormus or thallus. In the sporophyte generation, zygotes made by pollination of sperm nucleus and egg nucleus are germinated on gametophytes, thereby becoming sporophytes. Sporophytes create sporangia in which spores are created. New gametophytes are generated when spores germinate, and gametophytes live an autotrophic life as they have chloroplasts.
Most mosses grow on the ground, epiphytically on moist land, rocks, decayed trees and tree trunks, but some of them grow epiphytically on the leaves of broad-leaved trees. We can commonly see mosses around us, which mostly grows well in shaded and moist areas. Mosses also do not die out even in an extremely dry condition, and it undergoes photosynthesis as its resilience improves quickly with moisture supply (During, 1992; Oliver et al., 2005). Although there are slight differences among types of bryophytes, most mosses can contain moisture up to 50ŌĆō200% of dry weight (Schofield, 2001). Mosses not only can hold much moisture but also has high utilization value as indoor plants as they grow well in shades (Kim et al., 2009). Moreover, bryophytes have the ability to purify heavy metal underwater (Choi, 1992), and are also known to have the ability to remove toluene from indoor air pollutants (Kim et al., 2010). With such excellent functions, the utilization value of bryophytes is recently increasing as materials for indoor landscaping, especially wall planting and miniature landscape, and in some cases moss gardens are formed for landscaping, using moss as the main material.
Despite the increasing use of mosses, there are only a few studies on the cultivation conditions of some native mosses (Cho and Lee, 2013a, 2013b), but barely any research on the selection of native bryophytes according to the environmental factors. When the temperature drops significantly in winter like in Korea, mosses brown easily or stop growing. Thus, it is necessary to select moss species with great adaptability to low temperature, which can be easily recovered at normal temperature even though damaged by low temperature.
Therefore, this study was conducted to provide some basic data for use of native mosses by determining their growth responses at low temperature as well as their resilience at room temperature by using nine native moss species.
Total 9 species of mosses were used in this study: Atrichum undulatum (Hedw.) P. Beauv., Etodon luridus (Griff.) A. Jaeger, Marchantia polymorpha L., Thuidium cymbifolium (Mitt.), Myuroclada maximowiczii (G.G. Borshch.) Steere & W.B. Schofield, Bachythecium plumosum (Hedw.) Schimp, Plagiomnium cuspidatum (Hedw.) T.J. Kop, Hypnum plumaeforme Wilson, and Hypnum erectiusculum Sull. & Lesq. All mosses were collected from a mountain near Jinju in June with permission of the owner, which were then maintained and cultivated at a vinyl greenhouse in Gyeongnam National University of Science and Technology. Moss samples were sent to the National Institute of Biological Resources to verify the species, and the species were identified by a moss classification expert. Mosses were used by chopping them with a sharp steel frame (7cm2) and creating blocks in a fixed size. The mosses were placed on two layers of white non-woven fabric (10cm2) and put into a white plastic basket with many holes at the bottom. This basket is put into a plastic tray with 3cm edges on all four sides, and water was supplied through bottom watering.
To determine growth responses of mosses at low temperature, we set the plant growth temperature (JSPC-420C, JSR. INC., Korea) into two levels for the experiment: day/night temperature set at 15┬▒2/5┬▒1┬░C (16h/8h), and day/night temperature set at 5┬▒1┬░C(24h). For 8 weeks after low temperature treatment, we regularly examined the changes in moss block size and moss color. To determine the resilience of mosses when they are placed at room temperature after low temperature treatment, we set the plant growth temperature at 20┬▒2┬░C. We examined the changes in block area and color at one-week intervals. Light intensity for plant growth (PPFD) was set at 50┬▒3╬╝mol┬ĘmŌłÆ2┬ĘsŌłÆ1(16h), and relative humidity at 70┬▒3%.
The experiment began from the beginning of August, and we designed the experiment in a 10-times repetition using 10 blocks for each moss species. For 8 weeks after low temperature treatment, we examined the growth responses at low temperature. After that, the day/night temperature was reset at 20┬░C, and we examined their recovery for 6 weeks. Mosses have extremely small plant body, and thus there are many difficulties in examining growth. We took photographs regularly to investigate the changes in moss block area and moss color. Photographs were taken right before low temperature treatment, at 4 and 8 weeks after low temperature, and second, 4 and 6 at room temperature. With the photographs, we conducted image analysis using the Photoshop program (Photoshop CS6, Adobe, USA) to obtain moss block area and color.
The photos were opened on Photoshop program and the Magic Wand tool was used to select only moss, and the area of the selected part on Histogram was obtained in pixels (Fig. 1). At the same time, the moss color (red, green, and blue) value of the selected block was obtained. An analysis of variance was conducted on statistics using the SPSS 12.0 program (IBM, New York, USA), and significance among means was tested at the 5% significance level using DuncanŌĆÖs multiple range test.
At the low temperatures of 15/5┬░C or 5┬░C, all mosses showed a significant decrease in block area regardless of temperature, which dropped down to 50ŌĆō70% of the initial area at first one month (Fig. 2). After that, there was not much difference in moss block area for further one month. This suggests that when the minimum temperature drop to 5┬░C, mosses are stunted in growth or discolored, showing a rapid decrease of area, but after that they showed almost no growth. Mosses respond sensitively to environmental changes, and thus their growth is easily discontinued, and they enter dormancy if the weather becomes too dry or cold. Stunted growth of mosses at low temperature varied among species. Etodon luridus and Hypnum erectiusculum showed the least area decrease (Fig. 2B, 2I), whereas Marchantia polymorpha, Bachythecium plumosum, and Hypnum plumaeforme showed the most area decrease (Fig. 2C, 2F, 2H). The other five types were similar.
After low temperature treatment, the plant growth temperature was set to 20┬░C and the recovery degree of mosses was examined for 6 weeks. It was found that moss block area rather decreased in the first 2 weeks, showing approximately 20% decrease than the low temperature state (Fig. 2). After that, moss block area gradually increased over time. Moss block area rather decreased in the first 2 weeks even when growth temperature increased to 20┬░C, which is the optimum growth temperature of moss. It seems likely that any symptom of low temperature injury was not shown, because most mosses easily stop growth or begin dormancy at 5┬░C, but the symptoms of injury appeared on the surface after metabolic activities started at optimum growth temperature. The recovery of mosses at optimum growth temperature varied among moss species. Etodon luridus, Myuroclada maximowiczii, and Hypnum erectiusculum showed the most recovery in moss block area compared to other mosses (Fig. 2B, 2E, 2I). They showed 70ŌĆō80% recovery of the initial area at 6 weeks at room temperature. The mosses having the biggest injury at the low temperature and the least recovery at room temperature were Atrichum rundulatum, Marchantia polymorpha, Thuidium cymbifolium, and Hypnum plumaeforme, which were merely 50% of the initial area in 6th week at 20┬░C (Fig. 2A, 2C, 2D, 2H).
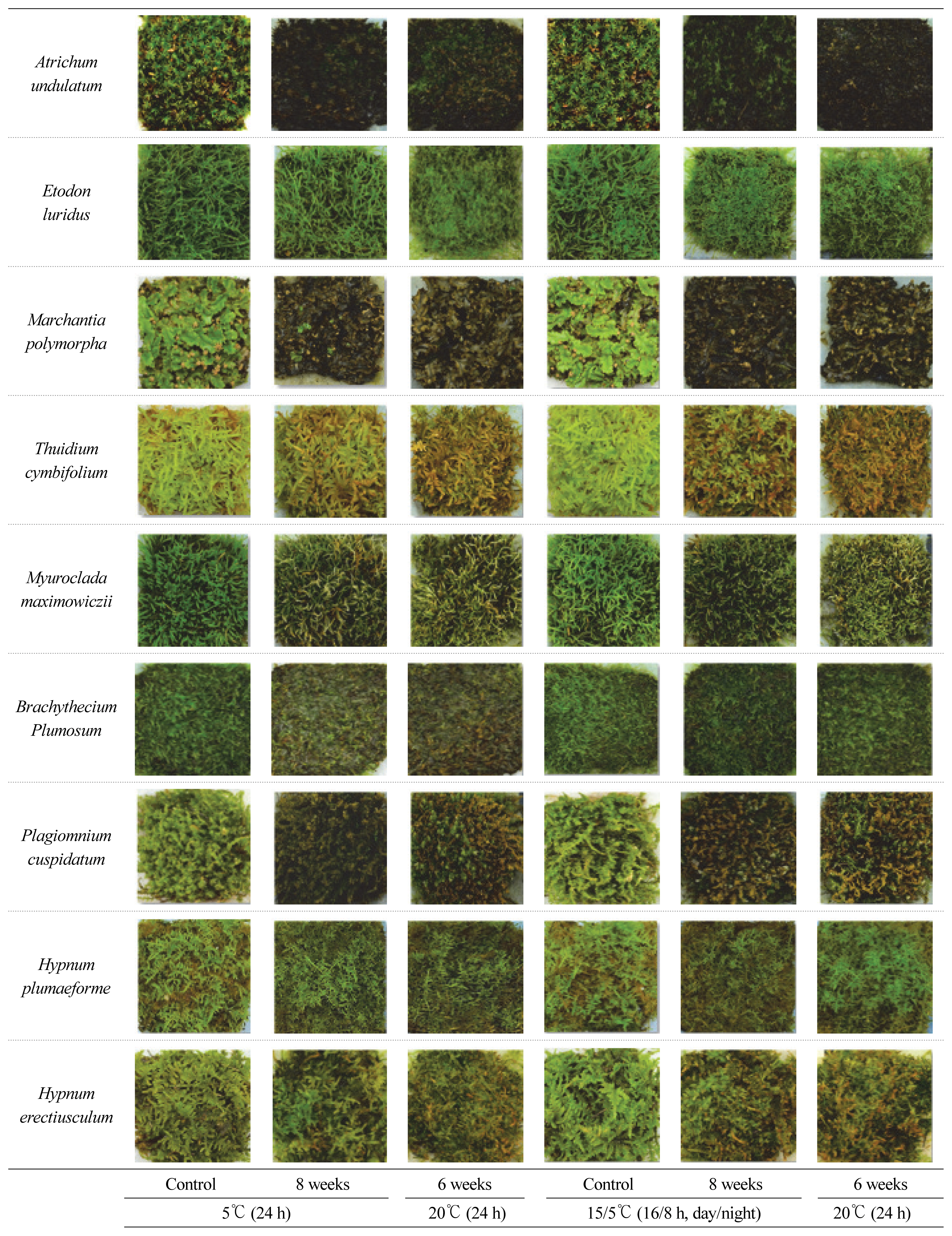
When mosses are used in indoor or outdoor landscaping, they look fresh in green and thus green mosses have high utilization value. However, mosses easily enter dormancy when the temperature drops in winter and the color turns brown, thus likely to lose ornamental value. Therefore, the ability of moss quickly to recover at room temperature is very important characteristics. Table 1 and Fig. 3 show the changes in moss color after low temperature treatment and at optimum growth temperature. When mosses are treated at low temperatures of 15/5┬░C (16/8h, day/night) or 5┬░C (24h), red, green and blue values all decreased greatly. All color values that decreased at 4 weeks of low temperature treatment regardless of the temperature remained almost the same by 8 weeks as well. After the temperature was transferred to optimum temperature later, the recovery of green varied among moss species. Red and blue did not show any correlation with moss block area or growth state, and only green showed a correlation with moss block area or growth state, which is why we will explain changes in green values here. In fact, it is more important to maintain greenness in reality as well.
At low temperature of 15/5┬░C, Etodon luridus showed 87% of initial value in green color, and 90% at room temperature. Hypnum plumaeforme also had 85% of the initial greenness at low temperature, and increased to 98% at room temperature, showing that it is well maintaining greenness despite the reduced area by low temperature and revealing more greenness at room temperature. Bachythecium plumosum decreased in greenness to 70% of initial value at low temperature, but recovered up to 90% at room temperature. Therefore, these three mosses are strong against low temperature and quickly recover at room temperature after low temperature treatment.
On the other hand, the species that showed the most remarkable decrease in greenness at low temperature were Atrichum undulatum and Marchantia polymorpha, which had their greenness drop to below 50% of the initial greenness. This suggests that even when the temperature setting was changed to room temperature, the greenness was not recovered and thus completely withered, or showed barely any recovery at all. Thuidium cymbifolium damaged by low temperature, failed to recover even at room temperature, and the greenness gradually decreased from 60% to 40%. The other three species such as Myuroclada maximowiczii, Plagiomnium cuspidatum, and Hypnum erectiusculum showed a moderate decrease in greenness at low temperature and moderate recovery of greenness at room temperature.
Treatment at 5┬░C (24 h) also showed similar results with 15/5┬░C temperature treatment. Etodon luridus and Hypnum plumaeforme showed the least decrease in greenness at low temperature, and showed high greenness at room temperature. Only Bachythecium plumosum showed little change in greenness at 15/5┬░C but showed decrease of up to 60% of the initial value, while also rarely showing any recovery. On the other hand, Atrichum undulatum, Marchantia polymorpha, and Plagiomnium cuspidatum showed decrease in greenness by 50% of the initial value, and there was barely any change in greenness even at room temperature. Thuidium cymbifolium, like at 15/5┬░C, showed continuous decrease in greenness even at room temperature.
This tendency was found more precisely in actual photographs (Fig. 3). Etodon luridus maintained greenness without any injury at the two low temperatures and retained vivid green at room temperature, showing greatest tolerance to cold. Bachythecium plumosum did not show severe injury at low temperature of 15/5┬░C, and also seemed healthy at room temperature. Hypnum plumaeforme showed decrease in greenness due to low temperature, but the green was vivid on living mosses, and showed more vivid greenness due to room temperature.
In summary of the moss block area, greenness and actual images, Etodon luridus that showed the least change in moss block area and also in green value (Table 1, Fig 3). Bachythecium plumosum showed a great decrease in moss block area at 15/5┬░Ct, but maintained the greenness without much decrease due to low temperature. These results indicated that these 2 species are relatively strong against low temperature. Hypnum plumaeforme showed a great decrease in moss area due to low temperature, but living mosses did not turn brown but showed vivid green, thereby maintaining ornamental value. Hypnum erectiusculum shown a little decrease in moss block area due to low temperature as well as high resilience, showed a significant decrease in greenness, indicating that it is not strong against low temperature.
On the other hand, Atrichum undulatum, Marchantia polymorpha, and Thuidium cymbifolium which showed a great decrease in moss area at low temperature and did not show a great increase in moss area at recovery temperature, had significantly lower greenness than other species, thereby proving to be sensitive to low temperature. The other species such as Myuroclada maximowiczii, Plagiomnium cuspidatum and Hypnum erectiusculum showed moderate responses low temperature.
Furness and Grime (1982) also examined growth responses to temperature by cultivating 40 species of bryophytes in various temperature ranges, and found out that most mosses showed the best growth at 15ŌĆō25┬░C, but only Dicranella palustris and Racomitrium lanuginosum grew well at the low temperature of 12ŌĆō13┬░C. More than half of the species showed a decrease in growth rates to below 50% at 5┬░C, indicating that responses to low temperature varied among species.
Atrichum undulatum has leaves that curl up when dry and uncurled when there is water, thereby having a great ornamental value (Choi, 1980), but it is sensitive to low temperature. Myuroclada maximowiczii (G.G. Borshch.) Steere & W.B. Schofield has round leaves and smooth stems and branches, thus suitable for indoor landscaping, but they stop growing and turn brown when the temperature drops to below 5┬░C in winter. Indoor or outdoor landscaping of building using mosses can reduce heating and cooling costs and alleviate the phenomenon of urban heat island (Lee et al., 2005), and thus use of mosses is expected to increase for roof or wall planting. However, when using mosses, it is important to choose the ones with high resistance to low temperature as well as resilience.
As a result of reviewing resistance of nine species of Korean native mosses to low temperature as well as their resilience at room temperature, it was found that three species such as Etodon luridus, Bachythecium plumosum, and Hypnum plumaeforme did not show a significant decrease in moss area and greenness even at low temperature compared to others, and their recovery was also quick at room temperature. On the other hand, three species such as Atrichum undulatum, Marchantia polymorpha, and Thuidium cymbifolium showed a significant decrease in moss area and greenness at low temperature, and little recovery at room temperature. Myuroclada maximowiczii, Plagiomnium cuspidatum, and Hypnum erectiusculum showed moderate responses to low temperature compared to the other six species. Therefore, the results above must be considered in using native mosses for roof or wall planting, and outdoor and indoor landscaping.
Fig.┬Ā1
Image analysis by Adobe Photoshop program. After opening a moss photograph, only moss block was selected by the magic wand (A). The moss block area was obtained by the pixel value and moss color was obtained by the medium value of each color (red, green, and blue) on the histogram window (B).
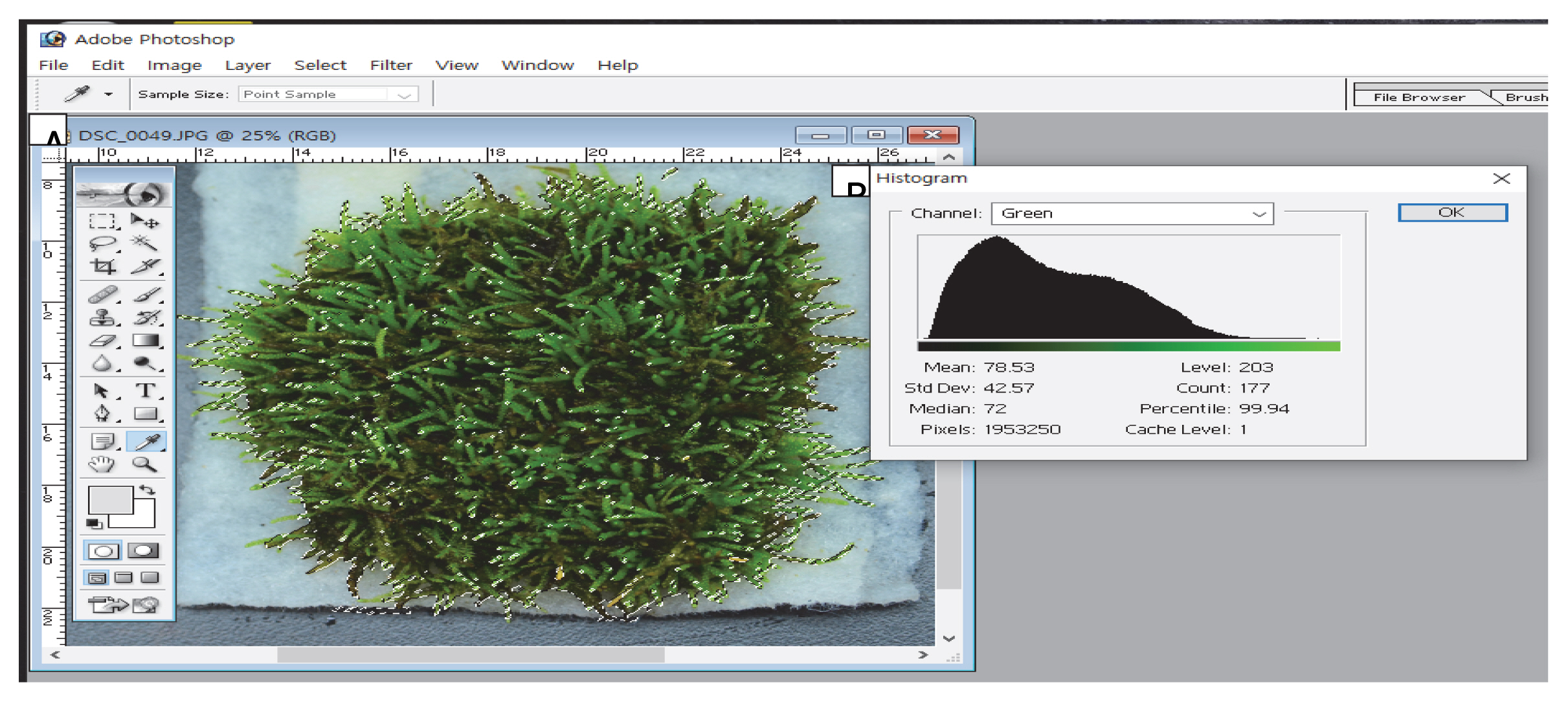
Fig.┬Ā2
Changes of moss block area during exposure to low temperature of 15/5┬░C (day/night, 16/8 h; gray circles) or 5┬░C (24 h; black circles) and during at 20┬░C (24 h). GLM repeated measures ANOVA was conducted before treatment(BT), 4, and 8 weeks during low temperature and 2, 4, and 6 weeks during recovery. Results are represented as the mean (n=10). (A) A. undulatum, (B) E. luridus, (C) M. polymorpha, (D) T. cymbifolium, (E) M. maximowiczii, (F) B. plumosum, (G) P. cuspidatum, (H) H. plumaeforme, (I) H. erectiusculum.

Fig.┬Ā3
Changes of moss color during exposure to low temperature of 15┬░C/5┬░C (16/8 h, day/night) or 5┬░C (24 h) and during recovery at 20┬░C (24 h).
Table┬Ā1
Changes of moss color during exposure to low temperatures (15/5┬░C, day/night, 18/6 h or 5┬░C, 24 h) and during recovery (20┬░C, 24 h)
| Temperature | Scientific name | Color | Low temperature | Recovery temperature | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 0 week | 4 weeks | 8 weeks | 2 weeks | 4 weeks | 6 weeks | |||
| 15┬░C/5┬░C (16h/8h) | Atrichum undulatum | Red | 100 | 36.8dz | 34.7d | 34.9c | 37.8d | 32.0c |
| Green | 100 | 41.7d | 45.1de | 47.6d | 47.9de | 46.5ef | ||
| Blue | 100 | 36.3d | 30.3d | 29.4d | 35.0d | 27.7e | ||
|
|
||||||||
| Etodon luridus | Red | 100 | 67.0b | 60.5bc | 58.4ab | 83.2a | 79.2a | |
| Green | 100 | 88.1a | 86.9a | 79.9bc | 91.3a | 86.5ab | ||
| Blue | 100 | 60.1b | 48.5bc | 52.8bc | 66.4b | 79.7ab | ||
|
|
||||||||
| Marchantia polymorpha | Red | 100 | 71.9ab | 59.9bc | 63.7ab | 47.8cd | 47.9cd | |
| Green | 100 | 57.4bcd | 43.5e | 47.8d | 36.7e | 38.2f | ||
| Blue | 100 | 80.3a | 73.9a | 79.3a | 77.8a | 83.4a | ||
|
|
||||||||
| Thuidium cymbifolium | Red | 100 | 80.3a | 82.8a | 73.2a | 72.3ab | 62.8ab | |
| Green | 100 | 60.1bc | 61.6c | 52.7d | 49.0de | 40.1f | ||
| Blue | 100 | 61.9b | 63.8b | 65.2b | 62.3bc | 44.3cd | ||
|
|
||||||||
| Myuroclada maximowiczii | Red | 100 | 54.0bc | 46.4cd | 49.8bc | 60.2bc | 60.3b | |
| Green | 100 | 70.5b | 67.2bc | 67.8bcd | 73.3bc | 88.3ab | ||
| Blue | 100 | 49.2c | 45.5cd | 50.9bc | 58.9bc | 63.2bc | ||
|
|
||||||||
| Brachythecium Plumosum | Red | 100 | 44.0cd | 52.0bc | 59.0ab | 62.5abc | 57.0b | |
| Green | 100 | 63.6bc | 69.7b | 89.8a | 89.0ab | 82.7ab | ||
| Blue | 100 | 38.5d | 36.0cd | 41.7c | 50.5c | 51.1cd | ||
|
|
||||||||
| Plagiomnium cuspidatum | Red | 100 | 53.3bc | 66.3ab | 59.3ab | 70.3ab | 64.3ab | |
| Green | 100 | 49.2cd | 55.7cd | 62.0cd | 68.8bcd | 61.5de | ||
| Blue | 100 | 46.0cd | 55.8d | 51.1bc | 61.4bc | 50.2cd | ||
|
|
||||||||
| Hypnum plumaeforme | Red | 100 | 40.6c | 57.8bc | 50.4bc | 72.4ab | 67.9a | |
| Green | 100 | 82.5a | 84.9ab | 84.8ab | 106.4a | 98.7a | ||
| Blue | 100 | 46.2cd | 61.2b | 49.5bc | 67.4b | 52.1cd | ||
|
|
||||||||
| Hypnum erectiusculum | Red | 100 | 50.0bc | 61.0bc | 61.8ab | 64.3abc | 65.4ab | |
| Green | 100 | 54.3bcd | 68.4bc | 67.6bcd | 65.4cd | 64.4cd | ||
| Blue | 100 | 44.1cd | 47.6c | 55.6bc | 45.4c | 39.5de | ||
|
|
||||||||
| 5┬░C (24h) | Atrichum undulatum | Red | 100 | 36.7d | 36.5d | 37.3d | 37.9d | 28.0e |
| Green | 100 | 41.2e | 43.4d | 46.3e | 45.7e | 34.5e | ||
| Blue | 100 | 37.5d | 37.7cd | 40.9c | 45.5cd | 29.9e | ||
|
|
||||||||
| Etodon luridus | Red | 100 | 60.0b | 82.1a | 74.3ab | 88.9a | 75.5a | |
| Green | 100 | 78.3a | 88.5a | 86.4a | 91.1a | 92.0a | ||
| Blue | 100 | 68.4bc | 64.9b | 72.3a | 88.2a | 88.0a | ||
|
|
||||||||
| Marchantia polymorpha | Red | 100 | 60.6b | 61.2bc | 61.0bc | 56.2bc | 46.4cd | |
| Green | 100 | 50.9cde | 49.2cd | 49.1de | 46.4e | 40.3d | ||
| Blue | 100 | 78.9ab | 78.0a | 77.3bc | 77.0b | 73.0b | ||
|
|
||||||||
| Thuidium cymbifolium | Red | 100 | 79.3a | 70.1ab | 84.0a | 72.7b | 62.b | |
| Green | 100 | 67.9abc | 57.8c | 58.1d | 57.0cde | 46.3ab | ||
| Blue | 100 | 88.8a | 81.5a | 70.4b | 81.1ab | 85.2ab | ||
|
|
||||||||
| Myuroclada maximowiczii | Red | 100 | 56.5bc | 45.1cd | 43.6cd | 48.9cd | 57.4bc | |
| Green | 100 | 66.3abc | 65.3b | 65.5c | 66.1cde | 70.9bc | ||
| Blue | 100 | 55.1bcd | 48.5cd | 54.7bc | 73.9b | 69.6bc | ||
|
|
||||||||
| Brachythecium Plumosum | Red | 100 | 41.0cd | 42.4d | 47.0cd | 51.3cd | 38.2de | |
| Green | 100 | 52.6bcd | 59.2bc | 67.1bc | 74.6c | 70.2bc | ||
| Blue | 100 | 36.9d | 30.8d | 35.5c | 46.7c | 51.2c | ||
|
|
||||||||
| Plagiomnium cuspidatum | Red | 100 | 43.1cd | 47.6cd | 50.9cd | 61.7bc | 59.1bc | |
| Green | 100 | 43.8de | 49.2cd | 54.2cd | 52.9de | 50.4cd | ||
| Blue | 100 | 42.5cd | 48.6cd | 56.3bc | 66.0bc | 78.9bc | ||
|
|
||||||||
| Hypnum plumaeforme | Red | 100 | 48.9bcd | 41.2d | 47.6cd | 52.0cd | 59.8bc | |
| Green | 100 | 68.3ab | 68.0b | 80.3b | 87.8b | 90.7a | ||
| Blue | 100 | 51.4bcd | 44.0cd | 54.2bc | 42.0cd | 49.7cd | ||
|
|
||||||||
| Hypnum erectiusculum | Red | 100 | 49.3bcd | 48.3cd | 49.2cd | 59.2bc | 63.3ab | |
| Green | 100 | 59.3bcd | 60.4bc | 60.8cd | 69.3bcd | 66.8c | ||
| Blue | 100 | 43.3cd | 39.5cd | 45.2c | 36.7d | 35.9d | ||
References
Cho, JS, CH Lee. 2013a. Effect of several cultivation condition on growth of Brachythecium rivulare and Myuroclada mazximoviczii. Korean J Plant Resour. 26(1):52-59.

Cho, JS, CH Lee. 2013b. Effect of several cultivation condition on growth of Plaglomnlum trichomanes and Atrichum undulatum. Flower Res J. 21(2):78-83.

Choi, DM 1980. Musci and Hepaticae. Illustrated flora and fauna of Korea 24:(pp. 403-422). Seoul, Korea: Ministry of Education.
Choi, YG 1992. Survey on the water quality and the heavy metal content of the fish, shellfish, moss and soil in Kum river. Master,s thesis. Kongju University, Gongju, Korea.
During, HJ 1992. Ecological classifications of bryophytes and lichens. In: Bates JW, Farmer AM, (Eds), Bryophytes and lichens in a changing environment (pp. 1-31). Oxford, England: Oxford Science Publications.

Furness, SB, JP Grime. 1982. Growth rate and temperature responses in bryophytes: II. A comparative study of species of contrasted ecology. J Ecol. 70(2):525-536. https://doi.org/10.2307/2259920

Kim, HG, KC Cho, IT Hwang, JB Seo, GY Gi, JG Kim. 2009. Study on available substrate for early planting Hypnum plumaeforme (Abstr.). Korean J Hortic Sci Technol. 27(Suppl I):141.
Kim, IH, KY Huh, MR Huh. 2010. Cold tolerance assessment of Sedum species for shallow-extensive green roof system. Korean J Hortic Sci Technol. 28(1):22-30.
Lee, EH, KY Kang, EJ Na. 2005. Analysis of trends in patent applications for rooftop greening techniques. J Korean Environ Restor Reveg Technol. 8(1):88-99.
National Institute of Biological Resources2015. A field guide to bryophytes in Korea Seoul, Korea: Geobook.
Oliver, MJ, J Velten, BD Mishler. 2005. Desiccation tolerance in bryophites: A reflection of the primitive strategy for plant survival in dehydrating habitats? Integr Comp Biol. 45(5):789-799. https://doi.org/10.1093/icb/45.5.788

Schofield, WB 2001. Introduction to bryology New York, NY: The Blackburn Press.
- TOOLS
-
METRICS

-
- 1 Crossref
- 2,013 View
- 30 Download
- Related articles in J. People Plants Environ.






