 |
 |
- Search
| J. People Plants Environ > Volume 25(6); 2022 > Article |
|
ABSTRACT
Background and objective: As the COVID-19 pandemic gripped the entire world and people found themselves spending more time at home, many households began gardening, and purchased various ornamental plants. Many cultivars of Hibiscus syriacus are grown for their showy flowers, or used as landscape shrubs. H. syriacus is generally known for its high rate of asexual reproduction. However, it is known that the effectiveness of propagation by cuttings can have big differences depending on internal and external factors. This study was conducted to determine the effects of optimal rooting bed soil composition and auxin on the rooting of H. syriacus cuttings.
Methods: Cuttings used in this study were from 17 cultivars. As types of bed soil for propagation by cutting, gardening bed soil, sand, mixed soil 1, and mixed soil 2 were used, and the plant growth regulators of IBA and NAA were applied at 500, 1000, and 1500 mg·L−1 concentrations.
Results: The rooting rate and number of roots were highest with the combination of perlite and vermiculite. On the other hand, the gardening bed soil showed an extremely low rooting percentage. The root growth was improved in most cultivars when treated by plant growth regulator. The highest rooting rate was verified at IBA 500 mg·L−1 treatment while the number of roots and root length showed good result in IBA 1500 mg·L−1 treatment.
Conclusion: In many cultivars, it was observed that the rooting rate and number of roots differed depending on the bed soil. The most suitable bed soil for the cuttings was a mixture of peat moss and vermiculite, and it was possible to increase the efficiency through treatment with a growth regulator, and the efficiency of IBA was better than that of NAA. However, it is necessary to identify which detailed growth regulator treatment is suitable for the root development of each cultivar, because plant growth regulator was less effective and even problematic in some cultivars.
Methods: Cuttings used in this study were from 17 cultivars. As types of bed soil for propagation by cutting, gardening bed soil, sand, mixed soil 1, and mixed soil 2 were used, and the plant growth regulators of IBA and NAA were applied at 500, 1000, and 1500 mg·L−1 concentrations.
Results: The rooting rate and number of roots were highest with the combination of perlite and vermiculite. On the other hand, the gardening bed soil showed an extremely low rooting percentage. The root growth was improved in most cultivars when treated by plant growth regulator. The highest rooting rate was verified at IBA 500 mg·L−1 treatment while the number of roots and root length showed good result in IBA 1500 mg·L−1 treatment.
Conclusion: In many cultivars, it was observed that the rooting rate and number of roots differed depending on the bed soil. The most suitable bed soil for the cuttings was a mixture of peat moss and vermiculite, and it was possible to increase the efficiency through treatment with a growth regulator, and the efficiency of IBA was better than that of NAA. However, it is necessary to identify which detailed growth regulator treatment is suitable for the root development of each cultivar, because plant growth regulator was less effective and even problematic in some cultivars.
As the COVID-19 pandemic gripped the entire world in the early part of 2020, many people found themselves quarantined at home for weeks, or even months. As people spent more time at home, the garden market grew in scale (Gabellini and Scaramuzzi, 2022). The propagation and growing of ornamental plants is one of the most profitable sectors of the garden market (Darras, 2020). This is why it is so important to constantly introduce new species and cultivars, particularly those with a long ornamental season and different flowering times (Wani et al., 2018).
Unlike most ornamental plants that bloom in spring, Hibiscus syriacus L. (Rose of Sharon), an important garden plant representative of summer, has nearly 3 months of flowering time from July to September, and there are more than 350 cultivars of this plant that have been developed worldwide (Lee et al., 2020).
H. syriacus is generally known to have high reproductive capacity and can be easily propagated through vegetative methods, such as cutting and grafting (Ha et al., 2013). However, the quality of the plants obtained through vegetative propagation techniques can vary greatly. Roots of certain cultivars do not develop sufficiently, thereby demanding additional treatment, such as grafting and sealed cutting (Souza et al., 2015).
Propagation through cuttings is mainly used to secure high quality plants and vegetative propagation of excellent cultivars. The technique is known for producing plants with highly variable quality, depending on internal (age, nutrition status, and hormone content), as well as external factors (temperature, humidity, and soil properties). In this case, soil properties and plant growth regulators can be manipulated to increase production efficiency (Hartmann et al. 2002).
The soil’s chemical and physical properties not only influence the rooting percentage and speed of root growth, but also directly mold the shape of roots (Miller and Jones, 1995). Usually, it is necessary to use one or a combination of various bed soils, such as vermiculite, peat moss, perlite, or coconut coir, to successfully stimulate root development. Plant growth regulators, such as auxin, cytokine, gibberellin, ethylene, and abscisic acid (ABA) show diverse effects on plant development, depending on the method of application and concentration used (Campbell et al., 1999). For this reason, it is important to control external factors according to the specific purpose in each case. Plant auxins are known to be involved in cell growth stimulation and promotion of root development through their enhancement of the number of roots and rooting percentage (Overvoorde et al., 2010).
The ‘Creation and Management of Forest Resources Act’ was amended in 2017 as social interest in H. syriacus rapidly increased, therefore many local governments and public organizations are planting H. syriacus pursuant to this Act (Kwon et al., 2017). Thus, it has become a priority to develop production technologies that can guarantee the stable distribution of high-quality cultivars of interest.
Previous studies on H. syriacus cutting propagation have focused on the environment required for the propagation of cuttings to increase the rooting percentage and the physiological characteristics for cuttings (Kwon et al., 2011). Some researchers have compared the root development and growth characteristics of various H. syriacus cultivars, and classified some cultivars that are difficult to propagate by cutting methods (Lee et al., 1998). However, there is little information about improving the production efficiency of such cultivars, which are not easily propagated through cuttings.
The present study compared the growth and development of cuttings cultured in soils with different properties and treated with plant growth regulators. The purpose of the study was to identify the optimal conditions for the stable propagation of H. syriacus cultivars, and thus solve the current problems associated with field-grown cuttings.
H. syriacus cuttings (Semi-hard wood, 10cm in length, 5 mm in diameter) collected from Jeon-wol mountain Rose of Sharon park (36°30′N, 127°17′E) in South Korea were provided by the City of Sejong in mid-March (Fig. 1). Cuttings in this study were of 17 domestic and foreign cultivars, including ‘Kojumong’ and ‘Coelestis,’ which recorded rooting percentages of under 40% in field cutting propagation surveys examined in 2021 (Table 1).
Thirty cuttings of every cultivar were planted on a cutting bed (52 × 31 × 10 cm) at 3 cm intervals. Cuttings were irrigated in a greenhouse at 60% humidity and an ambient temperature of 20 to 25°C. Two months later, we recorded rooting percentage, number of roots, root length, leaf length and width, and new twig length. The number of > 1 mm diameter roots was recorded to represent the number of roots; the length of the longest root was measured to represent root length; the longest and widest leaves were representative of leaf length and leaf width; and the longest twigs represented new twig length.
We investigated root and shoot growth of H. syriacus cuttings in various prepared mixtures of soils for propagation by cutting, including gardening bed soil (Hanareum, Shinsung, Korea), sand, mixed soil 1 (peat moss: vermiculite: granite, 1 : 1 : 1, v/v/v), and mixed soil 2 (perlite: vermiculite, 1 : 1, v/v).
The mixed soil 2, which promoted the highest rooting rate, was used as the basic culture medium for investigating reproduction differences according to the type and concentration of plant growth regulators. IBA (57310-25G-F, Sigma, USA) and NAA (N0640-25G, Sigma, USA) were diluted to 500, 1000, and 1500 mg·L-1, and the base of each cutting was dipped into an aqueous solution of auxins with corresponding concentrations for 10 mins.
In many cultivars, differences in rooting rate and number of roots depending on the bed soils were observed. However, leaf length and width and the length of new twigs and roots did not exhibit any significant difference in most cultivars. The rooting rate according to bed soil was highest in mixed soil 2. On the other hand, the gardening bed soil showed an extremely low rooting percentage. Even when roots emerged, the tips of the roots were rotten or showed poor growth. This soil type was thus considered unsuitable for the propagation of H. syriacus cuttings. Soffer and Burger (1988) and Yoshida et al. (1992) reported that the root growth of cuttings was affected by the physicochemical properties of the bed soil. In addition, the physical properties of the bed soil directly affect the growth of the root (Marin et al,, 2022). Therefore, it is considered that the good root growth in a mixed soil 2 in this experiment is due to it having better water retention and air permeability compared to other soils.
The number of roots was also higher in mixed soil 2.
The rooting rate and root growth of the H. syriacus cultivars being studied varied with the type of bed soil; however, most of the cultivars showed high rooting rates in the mixed soil. Some cultivars, including ‘Hanmaum’ and ‘Chungmu,’ showed considerable differences in rooting percentage depending on the bed soil. For this reason, it is highly important to choose a suitable bed soil for the successful propagation of cuttings. Conversely, neither ‘Sowol,’ ‘Hamabo’ or ‘Woodbridge’ germinated well in any bed soil, and showed a low rooting percentage (under 10%). These cultivars will require additional treatment for propagation by cutting (Fig. 2).
Eleven cultivars, including ‘Kojumong’, showed significant differences in the number of roots depending on the bed soil. However, other than these cultivars there were no significant differences. Outstanding root growth was observed in most cultivars in mixed soil, with the exception of ‘Coelestis.’ Notably, ‘Kojumong,’ ‘Hanmaum’ and ‘Pyonghwa’ developed the greatest number of roots in the peat moss: vermiculite: granite mixed soil. The remaining 8 cultivars produced the most roots in the perlite: vermiculite mixed soil (Table 2).
Some studies have examined the effects of soil type and composition on the root growth of horticultural plants, including rose and Hydrangea serrata. Similar to our findings, the observed rooting percentage was high in mixed soils, while relatively shorter and thicker roots were observed in the unmixed soil (Choi et al., 2000; Lee et al., 2009).
Bed soils consist of a solid, a gas, and an aqueous phase. A variety of substances can be combined in varying proportions to control aeration and water retention. When large particles are mixed together, soil porosity increases, allowing better aeration, while water retention decreases (Miller and Jones, 1995; Whitcomb, 2003). The gardening bed soil used in our experiments had a large water retention capacity. However, it is expected that the rooting percentage will be reduced due to poor aeration and high humidity, as the soil particles were small.
Hartmann et al. (2002) indicated that bed soil for propagation by cutting should have good water retention capacity, and thereby be able to provide sufficient moisture to help cuttings maintain their vitality until they develop roots. They also reported that good aeration is important to ensure a sufficient oxygen supply for the development of adventitious roots. Based on these findings, we believe that the mixture of vermiculite featuring high water retention capacity with granite or perlite will increase the aeration capacity for successful propagation of cuttings.
Among the surveyed characteristics, neither leaf length, leaf width, or length of new twig showed any difference related to the type or concentration of plant growth regulator treatment. In contrast, most cultivars showed significant differences in root growth characteristics, such as rooting rate, number of roots, and root length.
The average rooting rates for plant growth regulator type and concentration treatment were: NAA 500 mg·L−1 (45.3%), NAA 1000 mg·L−1 (46.2%), NAA 1500 mg·L−1 (55.9%), IBA 500 mg·L−1 (72.2%), IBA 1000 mg·L−1 (68.8%), and IBA 1500 mg·L−1 (60.4%).
A low-concentration treatment of IBA was most effective for stimulating the root growth of cuttings. IBA treatment promoted a higher rooting percentage compared to NAA. The rooting percentage tended to increase with decreasing IBA concentration and increasing NAA concentration (Table 3). These findings are similar to those reported by Lee and Hong (2003), which showed that IBA was most effective among the auxins to foster the root growth of H. hamabo ( Hamabo mallow ), and that the growth of plants treated with NAA was weaker compared to the controls. Al-Salem and Karam (2001) observed that auxin concentration had the greatest impact on root growth of steam cuttings in several tree species. They also found that neither the type nor the chemical composition of the auxins were significant. However, the H. syriacus cultivars used in the present study demonstrated varying degrees of root growth depending on the type of plant growth regulator administered. Therefore, we conclude that the selection of a plant growth regulator is important for effective propagation.
The number of roots and root length averaged 12.7 and 45 mm in the IBA 1500 mg·L−1 treatment, respectively. This treatment was identified as optimal for maximum enhancement of root growth in H. syriacus cuttings (Tables 4, 5). Treating cuttings with plant growth regulators increases the plant respiration, thereby facilitating the accumulation of amino acids and growth improvement (Strydom and Hartman, 1960). Hartmann et al. (2002) reported IBA as the best rooting stimulant with fast auxin activity and maximum enduring effect. In the present study, the variables associated with root growth responded positively to IBA treatment. Therefore, IBA is a better plant growth regulator than NAA to ensure the stable growth of cuttings.
‘Hamabo,’ ‘Sowol’ and ‘Woodbridge’ showed low root growth, which in turn caused an increased rooting rate depending on the plant growth regulator used. Considering this finding, the sample cultivars likely had difficulties in sprouting due to the lack of a sufficient auxin supply. Therefore, the problem of weak growth can probably be handled using an adequate plant growth regulator.
‘Hamabo,’ ‘Chungmu,’ ‘Hongsun’ and ‘Pyonghwa’ showed a remarkable drop in the root growth under the IBA 1500 mg·L−1 treatment. It is thought that an excessive level of auxin concentration which stimulates plant cell growth induces the synthesis of ethylene, consequently inhibiting growth. Barbez et al. (2017) reported that low auxin concentrations stimulate and high auxin concentrations inhibit root growth. Al-Saqri and Alderson (1996) found that high levels of plant growth regulators can severely damage plants. We also observed that high concentrations of IBA had negative impacts on the root growth of certain cultivars. Thus, it is most important to control the application of plant growth regulators at suitable concentration levels.
In this study, we collected some useful information concerning cultivars which typically experience difficulties in propagation through cuttings, including ‘Hamabo,’ ‘Sowol,’ ‘Woodbridge’ and ‘Pink giant.’ We subjected these and other cultivars to various treatment combinations using various bed soils and plant growth regulators. Several cultivars, however, showed poor responses to all of these treatments, and did not perform better than the controls. It is thought that each cultivar requires a different and a specific type of plant growth regulator at a specific concentration to be effective in fostering adequate root and shoot growth of the cuttings. For this reason, further research is required for propagation by cutting from more diverse H. syriacus cultivars.
In this study, the rooting rate and number of roots showed significant differences depending on the bed soil, but in most cultivars the length of the leaf, width of the leaf, length of new branches, and length of the roots did not show significant differences. Perlite: Vermiculite mixed soil was the most suitable bed soil for propagation of cuttings. On the other hand, it was observed that the gardening bed soil had a very low rooting rate and was unsuitable for planting H. syriacus due to rotting root tips or poor growth. Therefore, it is considered appropriate to mix vermiculite with granite or pearlite, which can increase the air permeability, in order to propagate the sapling of H. syriacus.
As a result of this experiment, the rooting rate of 5 cultivars (‘Hamabo’, ‘Sowol’, ‘Coelestis’, ‘Pink giant’, and ‘Woodbridge’) was found to be less than 30% in all bed soils, but could be increased through treatment with plant growth regulators. In most cultivars, IBA treatment was more effective than NAA treatment for root development.
H. syriacus is grown mainly as an ornamental landscape or garden plant. It is also cultivated as medicinal plant, because it contains useful substances for antioxidant, anticancer, and wound healing. Accordingly, the method of propagating H. syriacus, a biological resource with various useful values, is expected to have a wide range of applications.
Through this experiment, useful information was collected to secure H. syriacus cultivars that were difficult to propagate through cuttings. However, the treatment effect of growth regulators is observed to be insignificant or lower than that of the controls in some cultivars, so there is a difference among cultivars in the type and treatment concentration of growth regulators suitable for root growth. In the future, it is thought that detailed research on the asexual propagation of various H. syriacus cultivars will be needed.
Fig. 1
H. syriacus cutting collection site in South Korea. A. A map of the H. syriacus cutting collection site. The red triangle indicates Jeon-wol mountain Rose of Sharon park. B. H. syriacus plants in Rose of Sharon park. C. H. syriacus flower.
Fig. 2
Effect of bed soils on the rooting rate of the 17 H. syriacus cultivars. Mixed Soil 1 (peat moss : vermiculite : granite, 1 : 1 : 1, v/v/v); Mixed Soil 2 (perlite : vermiculite, 1 : 1, v/v).
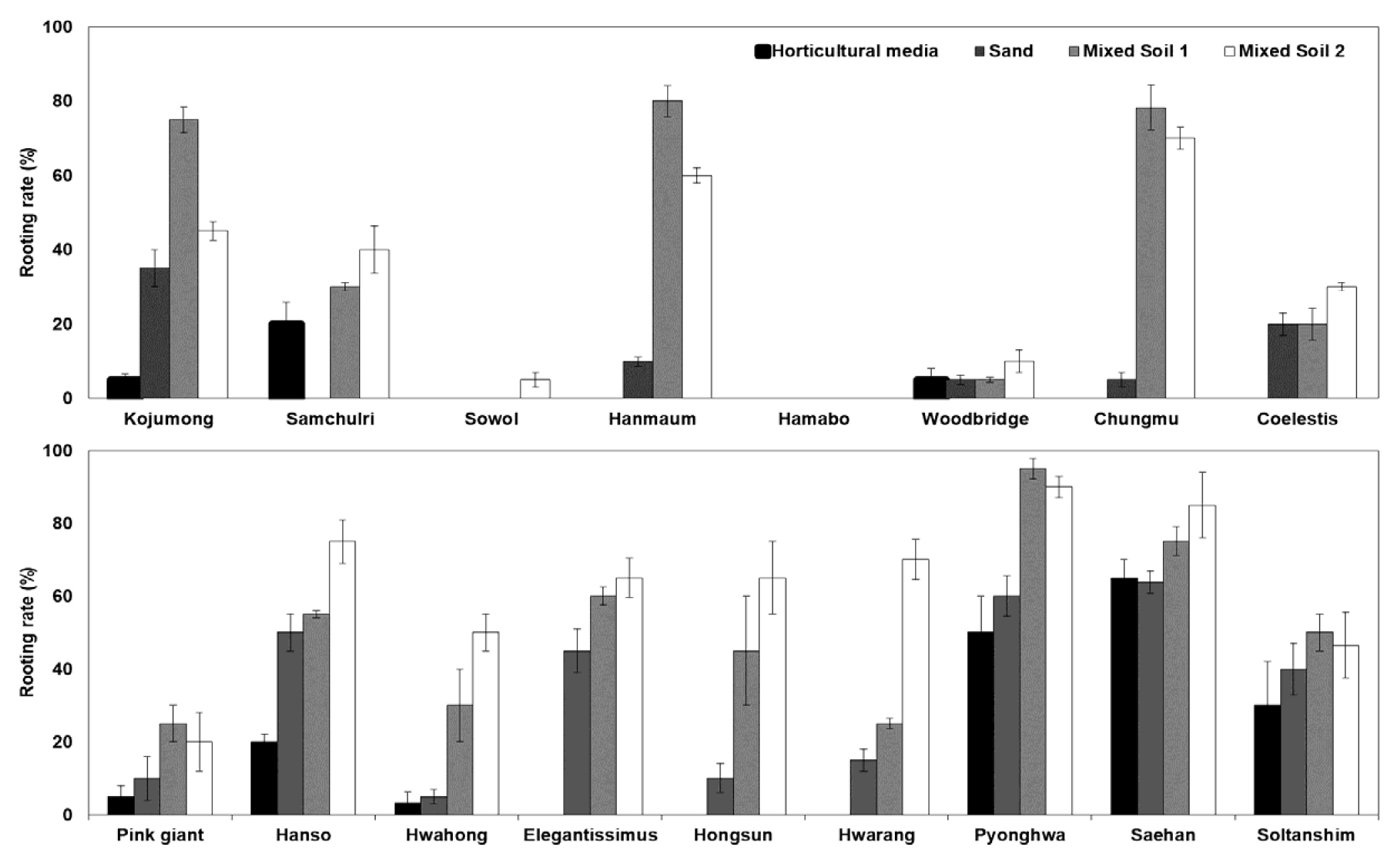
Table 1
Cultivar name and rooting rate of H. syriacus cultivars examined in this study. The cultivars used in this study had a low rooting rate of less than 40% in the field cutting propagation experiment in 2021
Table 2
Effect of bed soils on the number of roots of the 17 H. syriacus cultivars
| Cultivar name | Horticultural media | Sand | Mixed soil 1z | Mixed soil 2y |
|---|---|---|---|---|
| Kojumong | 1c* | 2.3bc | 6.9a | 4.8ab |
| Samchulri | 1 | 0 | 2.3 | 3 |
| Sowol | 0 | 0 | 0 | 1 |
| Hanmaum | 0c | 1bc | 3.8a | 2.7ab |
| Hamabo | 0 | 0 | 0 | 0 |
| Woodbridge | 1b | 2b | 1b | 7.5a |
| Chungmu | 0b | 1ab | 2.8ab | 4.6a |
| Coelestis | 0b | 2.3a | 1.3b | 2.0a |
| Pink giant | 3 | 1 | 3 | 4.5 |
| Hanso | 1.5b | 2.5b | 2.2b | 5.1a |
| Hwahong | 1b | 1b | 1.3b | 4.6a |
| Elegantissimus | 0c | 2.2bc | 2.8ab | 4.8a |
| Hongsun | 0b | 2.5ab | 3ab | 6.2a |
| Hwarang | 0b | 2.3b | 3.6ab | 7.3a |
| Pyonghwa | 1.9b | 5.7ab | 8.6a | 7.8a |
| Saehan | 3 | 3 | 3.9 | 3.7 |
| Soltanshim | 1.2 | 2 | 3.4 | 2.8 |
Table 3
Effect of growth regulator treatments on rooting rate of the 17 H. syriacus cultivars
Table 4
Effect of plant growth regulator treatments on the number of roots in the 17 H. syriacus cultivars
| Cultivar name | Control | NAA 500 mg·L−1 | NAA 1000 mg·L−1 | NAA 1500 mg·L−1 | IBA 500 mg·L−1 | IBA 1000 mg·L−1 | IBA 1500 mg·L−1 |
|---|---|---|---|---|---|---|---|
| Kojumong | 4.8b* | 2.9b | 3.6b | 3.9b | 3.4b | 4.8b | 8.0a |
| Samchulri | 3b | 4.6ab | 4.6ab | 2.9b | 2.3b | 2.7b | 7.3a |
| Sowol | 1 | 1.5 | 0 | 0 | 4.1 | 5.8 | 6.4 |
| Hanmaum | 2.7b | 5b | 2.1b | 5.9ab | 3.9b | 4.5b | 9.4a |
| Hamabo | 0 | 5 | 3 | 3.5 | 4.3 | 4.9 | 5.5 |
| Woodbridge | 7.5b | 4b | 2.7b | 2.7b | 5.4b | 11.6b | 27.3a |
| Chungmu | 4.6 | 3.7 | 3.4 | 2.8 | 4.8 | 5.8 | 3.3 |
| Coelesis | 2b | 2b | 2.8b | 1.9b | 2.6b | 2b | 7.6a |
| Pink giant | 4.5bc | 0c | 3.8bc | 3.3bc | 4.1bc | 8.8ab | 14.4a |
| Hanso | 5.1ab | 2.9b | 2.8b | 3.5b | 3.8b | 6.7a | 6.9a |
| Hwahong | 4.6b | 3b | 2.6b | 2.6b | 7.1b | 9.8ab | 17.8a |
| Elegantissimus | 4.8bc | 4.1bc | 5.7bc | 3.6c | 7.3b | 6.6bc | 13.3a |
| Hongsun | 6.2c | 6.2c | 6.4c | 4.1c | 8.3bc | 11.5b | 19.8a |
| Hwarang | 7.3bc | 2.8d | 4.1cd | 4.2cd | 5.9bcd | 8.5b | 17.3a |
| Pyonghwa | 7.8bc | 7.7bc | 7.3c | 6.3c | 12.5b | 20.0a | 22.9a |
| Saehan | 3.7d | 7.0bc | 6.1bcd | 4.4cd | 8.2b | 8.2b | 19.4a |
| Soltanshim | 2.8c | 4.9bc | 6.2b | 5.6b | 6.7b | 10.2a | 9.9a |
Table 5
Effect of plant growth regulator treatments on root length in the 17 H. syriacus cultivars
| Cultivar name | Control | NAA 500 mg·L−1 | NAA 1000 mg·L−1 | NAA 1500 mg·L−1 | IBA 500 mg·L−1 | IBA 1000 mg·L−1 | IBA 1500 mg·L−1 |
|---|---|---|---|---|---|---|---|
| Kojumong | 62.3 | 48.2 | 37.4 | 56.3 | 41.9 | 53.0 | 52.7 |
| Samchulri | 38.7 | 53.1 | 42.5 | 47.4 | 47.0 | 58.5 | 65.4 |
| Sowol | 7.9bc* | 27.4ab | 0c | 0c | 11.4bc | 38.2a | 43.7a |
| Hanmaum | 12.0b | 28.2ba | 16.5b | 27.7ba | 30.2ba | 16.5b | 43.9a |
| Hamabo | 0 | 37.7 | 34.1 | 35.7 | 35.4 | 42.9 | 30.0 |
| Woodbridge | 53.0ab | 30.5b | 23.9b | 28.4b | 20.0b | 47.4ab | 66.6a |
| Chungmu | 30.5 | 39.1 | 25.7 | 18.1 | 36.2 | 36.3 | 29.5 |
| Coelesis | 41.7 | 38.8 | 60.6 | 45.9 | 31.5 | 38.0 | 48.8 |
| Pink giant | 53.1a | 0b | 34.1a | 36.9a | 48.1a | 57.0a | 55.5a |
| Hanso | 40.5 | 35.0 | 31.6 | 28.8 | 31.3 | 28.3 | 35.5 |
| Hwahong | 68.2a | 17.6c | 43.3b | 75.5a | 18.3c | 42.2b | 38.8b |
| Elegantissimus | 43.6 | 37.1 | 43.5 | 53.5 | 47.2 | 53.0 | 45.7 |
| Hongsun | 70.0a | 31.6b | 32.1b | 47.9ab | 41.4b | 45.8b | 42.1b |
| Hwarang | 33.0a | 24.1ab | 16.3b | 31.8a | 22.9ab | 35.0a | 34.4a |
| Pyonghwa | 56.0ab | 55.8ab | 45.4b | 67.5a | 43.6b | 45.8b | 39.8b |
| Saehan | 36.7bc | 54.3a | 31.6c | 46.7ab | 46.5ab | 53.4a | 53.1a |
| Soltanshim | 44.6ab | 50.8ab | 34.3b | 63.9a | 45.1ab | 61.8a | 39.7b |
References
Al-Salem, MM, NS Karam. 2001. Auxin, wounding, and propagation medium affect rooting response of stem cuttings of Arbutus andrachne. HortScince. 36:976-978.

Al-Saqri, F, PG Alderson. 1996. Effect of IBA, cutting type and rooting media on rooting of Rosa centifolia. Journal of Horticultural Science. 71(5):729-737. https://doi.org/10.1080/14620316.1996.11515453

Barbez, E, K Dünser, A Gaidora, T Lendl, W Busch. 2017. Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 114(24):E4884-E4893. https://doi.org/10.1073/pnas.1613499114



Campbell, NA, JB Reece, LG Mitchell. 1999. Biology (5th ed.). CA, USA: Addison Wesley Longman, Inc Menlo Park, Calif.
Choi, BJ, CK Sang, EJ Choi, SA Noh. 2000. Effect of rooting media on rooting and root growth of rose cutting. Horticultural Science & Technology. 18(6):819-822.
Darras, AI 2020. Implementation of sustainable practices to ornamental plant cultivation worldwide: A critical review. Agronomy. 10(10):1570.https://doi.org/10.3390/agronomy10101570

Gabellini, S, S Scaramuzzi. 2022. Evolving consumption trends, marketing strategies, and governance settings in ornamental horticulture: A grey literature review. Horticulturae. 8(3):234.https://doi.org/10.3390/horticulturae8030234

Hartmann, HT, DE Kester, FT Davies, RL Geneve. 2002. Plant propagation: Principles and practices (7th ed.). NJ, USA: Prentice-Hall Inc.
Kwon, HY, SH Kim, MS Jung, SL Hwang. 2011. Growth and flowering characteristics of rooted cuttings of Hibiscus syriacus L. clutivars. Horticultural Science Technology (pp. 52 p.
Kwon, HY, SH Kim, JI Nam. 2017. Development of Hibiscus syriacus ‘Danna’ with small and vivid red eye spot on bright pink petals. Flower Research Journal. 25(1):35-40. https://doi.org/10.11623/frj.2017.25.1.06

Lee, HS, JS Lee, BW Kwack. 1998. Characteristics of rooting and shoot growth influenced by cultivar and flower types in Hibiscus syriacus hardwood cutting. Horticultural Science & Technology. 16(4):528-530.
Lee, JS, J Hong. 2003. Effect of auxins on rooting in leaf cutting of Hibiscus hamabo. Journal of the Korean Society of Environmental Restoration Technology. 6(6):25-28.
Lee, JS, SM Yoon, JH Kim, JY Sim. 2020. Global planting status of Korean Hibiscus (Hibiscus syriacus L) as a garden plant. Flower Research Journal. 28(1):1-7.
Lee, SY, NH Yun, JH Gu, SJ Jung, KJ Kim, JC Rhee, TJ Lee, JS Lee. 2009. Effect of leaf number and rooting media on adventitious rooting of softwood cuttings in native Hydrangea serrata for acuminata. Horticultural Science & Technology. 27(2):199-204.
Leonardi, C, A Ruggeri, S Malta. 2001. Hormone effects on in vitro proliferation and rooting of Grevillea explants. Scientia Horticulturae. 90(3–4):335-341. https://doi.org/10.1016/S0304-4238(01)00228-X

Ludwig-Muller, J 2000. Indole-3-butyric acid in plant growth and development. Plant Growth Regulation. 32:219-230.
Marin, M, PD Hallett, DS Feeney, LK Brown, M Naveed, N Koebernick, S Ruiz, AG Bengough, T Roose, TS George. 2022. Impact of root hairs on microscale soil physical properties in the field. Plant and Soil. 476:491-509. https://doi.org/10.1007/s11104-022-05530-1



Miller, JH, N Jones. 1995. Organic and compost-based growing media for tree seedling nurseries. World Bank Tech. Pap. No. 264 Forestry Series The World Bank. Washington, DC, USA: (pp. 75 p.
Overvoorde, P, H Fukaki, T Beeckman. 2010. Auxin control of root development. Cold Spring Harb Perspect Biol. 2(6):a001537.https://doi.org/10.1101/cshperspect.a001537



Soffer, H, DW Burger. 1988. Effects of dissolved oxygen concentrations in aerohydroponics on the formation and growth of adventitious roots. Journal of the American Society for Horticultural Science. 113(2):218-221. https://doi.org/10.21273/JASHS.113.2.218

Souza, SR, MZB Cavalcante, MPD Lima, TF Alixandre, RT Nascimento. 2015. Vegetative propagation of Hibiscus with different types of cuttings and IBA concentrations. Comunicata Scientiae Horticultural Journal. 6(3):291-296. https://doi.org/10.14295/cs.v6i3.679

Strydom, DK, HT Hartmann. 1960. Absorption, distribution and destruction of indole acetic acid in plum stem cuttings. Plant Physiology. 35(4):435-442. https://doi.org/10.1104/pp.35.4.435



Wani, MA, IT Nazki, A Din, S Iqbal, SA Wani, FU Khan. 2018. Floriculture sustainability initiative: The dawn of new era. Sustainable Agriculture Reviews Springer. Cham, Switzerland: 27:(pp. 91-127).

Whitcomb, CE 2003. Plant production in containers II Lacebark Publications. Stillwater, Oklahoma, USA:
Yoshida, H, T Hayashi, T Harada, K Konishi. 1992. Effect of medium composition and pretreatment on rooting of plug nursery plant. International Symposium on Transplant Production Systems. 319:441-446. https://doi.org/10.17660/ActaHortic.1992.319.69

- TOOLS
-
METRICS

-
- 0 Crossref
- 1,156 View
- 16 Download
- Related articles in J. People Plants Environ.
-
Effect of Slow Release Fertilizers on the Growth of Vegetables2014 April;17(2)






