Assessment of Adequate Volumetric Water Content for Managing Farfugium japonicum (L.) Kitam. in the Indoor Gardens
- ParkSo Hyun, ParkHyo Geun, KwonHyuck-Hwan, LeeKyung Mee
- Received November 20, 2023; Revised December 6, 2023; Accepted December 18, 2023;
- ABSTRACT
-
- Background and objective: The present research study aimed to investigate the optimum substrate volumetric water content (VWC) needed for Farfugium japonicum (L.) Kitam. to maintain its performance in the indoor garden.
- Methods: The threshold levels of substrate VWC were evaluated as 0.1, 0.2, 0.3 or 0.4 m3·m−3 using an automated irrigation system with capacitance soil moisture sensors and datalogger. Plant growth and physiological responses were measured and analyzed at 10 weeks after treatment. Plant growth was investigated by measuring survival rate, plant height, leaf length, leaf width, leaf number, root length, fresh and dry weight of shoot and root. Physiological responses were observed by analyzing antioxidant enzymes (SOD, POD) and antioxidants (MDA, proline), maximum quantum yield (Fv/Fm) and total chlorophyll contents.
- Results: The results of drought tolerance measurement by the treatment group showed that the overall growth was the highest in the 0.3 and 0.4 m3·m−3 treatment plot and the lowest in the 0.1 m3·m−3 treatment plot. Under the drought-stressed condition of the 0.2 m3·m−3 treatment, growth was not as efficient as the 0.3 and 0.4 m3·m−3 treatments due to the relatively higher moisture content, but there was no statistical difference between the 0.3 and 0.4 m3·m−3 treatments in the maximum quantum yield (Fv/Fm) and also total chlorophyll content. Furthermore, for antioxidant activities, SOD, POD, and MDA showed high values, but no statistical differences were found among treatments except for the 0.1 m3·m−3 treatment, which was under severe drought stress in terms of proline.
- Conclusion: Overall, the results indicate that Farfugium japonicum (L.) Kitam., has a tolerance to drought, and for managing indoor gardens the adequate soil moisture content for the growth is at least 0.2 m3·m−3 assuming the use of identical substrate in this experiment.
- Introduction
- Introduction
Generally, light conditions and temperature-humidity levels are the most crucial factors in plant growth. Indoor environments usually are unfavorable towards plant growth, due to constraints including low light levels, constant temperatures throughout the year which disturbs dormancy, low humidity which causes desiccation stress, low air movement which leads to reduced photosynthesis and nutrient absorption (Thongbai et al., 2010; Kitaya, 2015), and limited root run and constraints in nutrients and moisture held by the substrate (Poorter et al., 2012). However, indoor spaces, typically designed for human comfort, must maintain consistent temperatures around 20–25°C, and their structure usually limits light transmission in inevitable fashion. This leaves soil moisture the most controllable factor in plant cultivation and exhibition in indoor spaces (Madzhi, 2021; Nam et al., 2018). The most critical aspect of maintaining the aesthetic value of plants in relation to soil moisture is the regulation of soil moisture in the substrate to prevent the occurrence of drought stress.In the field of horticulture, various studies have been conducted to achieve efficient control of soil moisture content. Some research took approaches based on substrate properties, such as using soil moisturizing agents to enhance soil moisture retention (Kwon et al., 2022). Studies have also been carried out to determine the optimal irrigation timing based on the measurement of volumetric water content (VWC) using automated irrigation systems (Nam et al., 2017; Nam et al., 2018). In the subject of smart farming, technologies using Information and Communication Technology (ICT) have been introduced to maximize production by increasing water use efficiency and detecting and addressing anomalies in soil moisture, pH, and soil types (Jung and Lee, 2021; Ramachandran et al., 2018). Utilizing ICT automatic irrigation systems in gardens would not only enhance irrigation efficiency in indoor garden management, but also reduce input and budget required for landscape maintenance.For maintenance of normal growth and aesthetic value under indoor environments, a plant must be capable of adapting to low-light conditions by maintaining positive assimilation rate by efficient light capturing or conservation of photosynthates (Middleton, 2001). In addition, they should possess features such as minimal or shallow dormancy and leaf traits that confer tolerance to dry atmospheric conditions including reduced leaf area, hairiness, glaucousness, and thick cuticle layer (Wang et al., 2021). Plants with these characteristics are typically found in warm temperate and subtropical broadleaf evergreen forests (Ferreira et al., 2019).The genus Farfugium, belonging to the family Asteraceae, includes two species distributed in East Asia (Song, 2004). F. japonicum (L.) Kitam., commonly known as leopard plant, is a perennial evergreen herb native to Japan, South Korea, Taiwan, and China. In South Korea, they are distributed along the coastal areas and island regions in the southern part of the peninsula. They are often found in the understory of coastal forests composed of broadleaf evergreen trees. Leopard plants are widely used in gardens (Lee and Oh, 2022; Jung et al., 2008), due to their high shade tolerance which allows them to thrive in shade and partial shade (Kim et al., 2008), and high aesthetic value from their large, round leaves, glossy from their thick cuticle layers, and bright yellow flowers in autumn (Flora of China, 2008). Moreover, they are recognized for their effectiveness in formaldehyde and fine particle removal, making them popular choices for indoor gardens in office spaces (NIHHS, 2006).For effective moisture management, accumulating data on the minimum volumetric water content conditions necessary for maintenance of the aesthetic value of plants used in indoor gardens is required. Therefore, in this study we sought to determine the adequate soil moisture conditions for the native ornamental plant F. japonicum in indoor garden settings utilizing an automatic irrigation system.
- Research Methods
- Research Methods
- Materials for experiment
- Materials for experiment
F. japonicum used in this study was obtained in October 2022 from Sannae Botanical Garden located in Cheonan, Chungcheongnam-do. Certain individuals were selected for 2 weeks from April 3 to 17, 2023 and acclimatized them in a round 12 cm-diameter pot (820 ml) filled with a horticultural substrate (Baroker, SeoulBio, eumseong, Korea).- Experiment design
- Experiment design
The experiment was conducted in an indoor environment in Korea National Arboretum located in Pocheon, Gyeonggi-do. For artificial lighting, white: red (1:1) LED (Satellite Sunshine, Sekwang Electronic, Korea) was installed, and luminous intensity remained consistent at 48 μmol·m−2 ·s−1 and the photoperiod was set at 16 hours. Temperature and humidity were measured for one hour each day using the HOBO sensor (HOBO U23 pro v2, Onset, Boume, MA, USA), and the average temperature and humidity during the experiment were 22.1±6.3°C and 81.9±18.3%.Hyprop (UMS, Munich, Germany) was used to measure the water holding capacity of the horticultural substrate used in the experiment, and volumetric water content (VWC, v/v, m3·m−3) was measured in frequency domain reflectometry (FDR) using a soil moisture sensor (EC-5, Decagon devices, Pullman Washington, USA). For soil moisture sensor correction formula, VWC = 0.1595 × mV - 46.786, R2 = 0.97 was used. Three soil moisture sensors per treatment were inserted into the pot, and the plants were watered by setting the mean as the representative value. For automated irrigation, the measures of VWC were transmitted to the data logger (CR1000, Campbell Scientific, Logan Utah, USA), designed for the water to come out of the dropping pipette (dropper) inserted in the pot through an electromagnetic valve when VWC is below the set values. Here, the set values of VWC were in 4 levels (Fig. 1), the 0.4 m3·m−3treatment was in the range of effective moisture utilization (pF 1.0 - 1.7) and the 0.3 m3·m−3 treatment was in the range of moisture utilization was not smooth (pF 1.7 – 2.0), and the 0.2 m3·m−3 treatment was in dry state, and the 0.1 m3·m−3treatment was in extremely dry state, obtained by calculating the range of easily available water (EAW) and water buffering capacity (WBC). It was confirmed that the set values were maintained during the experiment (Fig. 2).The experiment was conducted for 10 weeks from May 15 to July 21, 2023 and randomly assigned, using 5 individuals of F. japonicum for each treatment.- Survival rate and growth investigation
- Survival rate and growth investigation
To examine survival rate and growth changes in F. japonicum due to drought stress, this study measured plant length, leaf length, leaf width, leaf number, root length, shoot and root fresh weight, and dry weight after 10 weeks. Plant length was measured from the soil surface to the apex of the plant, and leaf length and leaf width were measured from the largest leaf. Shoot and root fresh weight was measured using an electronic scale (Shimadzu Analytical Balance AUW2200, Shimadzu, Japan) immediately after the experiment ended, and dry weight was measured after drying in a constant temperature drying oven at 70°C for 72 hours.- Physiochemical response analysis
- Physiochemical response analysis
Specimen preparation
Specimen preparation
For analyze Antioxidant activities, the harvested plants were rapidly cooled using liquid nitrogen and stored in a deep-freezer set at −70°C. The frozen specimen was freeze-dried using a freeze dryer and then powdered. 20 mg of the powdered specimen was mixed with 2 ml of 90% H2O-methanol solvent using Voltex Mixer (SI-0246A, Coleparmer, USA). After that, the supernatant was obtained by sonication at 40°C for 40 minutes in Ultrasonic Bath (powersonic420, Hwashin Tech Co., Ltd., Korea) and then centrifuged at 15000 rpm for 5 minutes (Smart 15 plus, Hanil, Korea).Antioxidant enzyme analysis
Antioxidant enzyme analysis
To check the antioxidant enzyme reaction of Farfugium japonicum (L.) Kitam. due to drought stress, superoxide dismutase (SOD) and guaiacol peroxidase (POD) contents were measured 10 weeks after the experiment began.The enzyme extract prepared as follows to measure SOD and POD. All processes were carried out by maintaining cold conditions. 20 mg of the biological specimen was mixed in 50 mM pH 7.0 sodium phosphate buffer (2 ml), and cell lysis was performed by repeating freezing and thawing 3 times using liquid nitrogen and ice/water as explained by Shehadul et al. (2017). After that, the supernatant was separated and used for analysis after centrifugation at 4°C and 15,000 rpm for 5 minutes.SOD activity was measured by modifying the method by Gupta et al. (1993). The reaction mixture was prepared by thoroughly mixing 50 mM pH 7.0 sodium phosphate (93.5 μL), 0.1 M methionine (52 μL), 2.5 mM NBT (24.5 μL), 10 mM EDTA (2 μL), and 0.5 mM riboflavin (8 μL). The control was the one without the enzyme extract, and light was blocked after exposing to PPFD 50 μmol· m−2 ·s−1 of LED light along with the specimen. Absorbance was measured at 560 nm, and the amount of enzymes causing the SOD activity to reduce NBT by 50% was used as the unit for the following formula, expressing SOD activity as unit mg−1 DW. Blank had no enzyme extract in the reaction mixture, and absorbance was measured after being stored in the dark, and thermal equilibrium state was examined.• SOD (unit mg−1 FW) = unit ml−1/enzyme (mg·ml−1)POD activity was measured by modifying the method by Rao et al. (1996). The enzyme extract (20 μL) was put into the reaction mixture made by mixing 40 mM pH 6.1 sodium phosphate buffer (66.6 μL), 20 mM guaiacol (80 μL), and 3% H2O2 (33.3 μL), and absorbance was measured at 470 nm every 10 seconds, and this was applied to the following formula to calculate POD activity as μmol· min−1 ·mg−1 DW. Blank had no enzyme extract in the reaction mixture, and thermal equilibrium state was examined as a result of measuring absorbance.• POD (μmol·min−1 ·mg−1 FW) = unit ml−1/enzyme (mg·ml−1)Antioxidant analysis
Antioxidant analysis
Malondiadehyde (MDA) and proline were analyzed to examine the antioxidant expression of Farfugium japonicum (L.) Kitam. due to drought stress.MDA analysis was conducted by modifying the analysis method by Du and Bramlage (1992). 0.15 g of plant specimen was collected each, ground in liquid nitrogen, stored cold at 4°C, and homogenized by adding 70% ethanol. After that, it was centrifuged at 4°C and 15,000 × g for 15 minutes using a centrifuge (M15R, Hanil Scientific lnc., Korea). 2 ml of the supernatant of the specimen, 2 ml of 20.0% trichloroacetic acid (TCA), 1 ml of 0.67% thiobarbituric acid (TBA) dissolved in 20% TCA, and 50 μL of 100 mM butylated hydroxytoluene were added, which were shaken at 95°C for 15 minutes in a constant temperature water tank (WB-11, DAIHAN Scientific Co., Ltd., Korea) and then cooled for use. Then, the solution containing lipid peroxide was centrifuged at 15,000 × g for 15 minutes, and the absorbance of the supernatant was measured at 450 nm, 532 nm, and 600 nm using a spectrophotometer (X-ma 1200, Human, Seoul, Korea).Proline analysis was conducted using the method by Bates et al. (1973). 0.5 g of the leaves were ground using liquid nitrogen, homogenized by adding 10 ml of 3.0% sulfosalicylic acid, and then centrifuged at 4°C for 4,000 × 10 minutes. 1 ml of supernatant, 1 ml of acid-ninhydrin, and 1 ml of acetic acid were mixed, which were shaken at 95°C for 60 minutes in a constant temperature water tank and then cooled. Acid-ninhydrin was prepared by mixing 60 ml of acetic acid and 40 ml of 6 M phosphoric acid, adding 2.5 g of ninhydrin, and then stirring. After adding 3 ml of toluene to the solution where the reaction was completed, the mixture was stirred for 30 seconds and stopped at room temperature for 10 minutes to separate the color development of the solution. To quantify proline, the value of each reaction specimen was measured at an absorbance of 520 nm using toluene as the blank value.Measurement of chlorophyll fluorescence and chlorophyll content
Measurement of chlorophyll fluorescence and chlorophyll content
Chlorophyll fluorescence was analyzed by selecting 6 leaves per treatment at 0, 1, 6, and 10 weeks during the experiment and measuring maximum quantum yield (Fv/Fm) using a chlorophyll fluorometer (PAM-2500, Heinz Walz GmbH, Effeltrich, Germany) after 30 minutes of dark adaptation.Extraction and measurement of chlorophyll content were performed by modifying the method by Hiscox and Israelstam (1978). For the specimen, 0.25 g of leaves per treatment were ground and immersed in 5 ml of dimethyl sulfoxide (DMSO), and the pigment was extracted for 6 hours under dark conditions at 60°C, after which the chlorophyll content was measured at wavelengths of 663 nm and 645 nm using a spectrophotometer (X-ma 1200, Human, Seoul, Korea), and total chlorophyll content was obtained with reference to Arnon (1949) and Mackinney (1941).
- Statistical analysis
- Statistical analysis
For statistical analysis, analysis of variance was conducted using SPSS (version 20, IBM Corporation, Armonk, NY, USA), and a post-hoc test was conducted using the Duncan test (p≤0.05) when there were significant statistical differences. The graphs were created using SigmaPlot (version 12.5, SYSTAT Software, Chicago, IL, USA).
- Results and Discussion
- Results and Discussion
- Survival rate and growth changes in F. japonicum according to VWC
- Survival rate and growth changes in F. japonicum according to VWC
The growth data measured according to volumetric water content treatments (θ = 0.1, 0.2, 0.3, 0.4 m3 ·m−3) revealed that the survival rate in the 0.1 m3 ·m−3 treatment plot was the lowest at 46.7%, while the treatment plots excluding 0.1 m3 ·m−3 showed a survival rate of over 80%. For plant height, excluding the 0.1 m3 ·m−3 treatment plot with a height of 8.0 cm, there was no statistically significant difference between the 0.2, 0.3, and 0.4 m3 ·m−3 treatment plots. The treatment plot with the highest plant height was the 0.4 m3·m−3, measuring 12.2 cm. In terms of leaf length, the 0.1 and 0.2 m3 ·m−3 treatment plots differed from the 0.3 and 0.4 m3 ·m−3 treatment plots, with the 0.4 m3 ·m−3 plot measuring 12.2 cm, approximately twice the 6.9 cm measured in the 0.1 m3 ·m−3. Leaf width did not show statistically significant differences between the treatment plots. Leaf number was highest in the 0.4 m3 ·m−3 treatment plot at 2.3 leaves, while the 0.1 m3 ·m−3 treatment plot measured 1.4 leaves, with no statistically significant differences observed for treatments above 0.2 m3 ·m−3. Root length in the 0.1 m3 ·m−3 treatment plot was the lowest at 7.1 cm, while the remaining treatment plots showed obvious difference, measuring over 23.0 cm (Table 1).- Physiochemical response of F. japonicum due to drought stress
- Physiochemical response of F. japonicum due to drought stress
Antioxidant analysis
Antioxidant analysis
SOD was measured at 0.086 unit mg-1 FW in the 0.3 m3 ·m−3 treatment plot, which decreased by about 3.2 times compared to 0.278 unit mg-1 FW in the 0.1 m3 ·m−3 treatment plot. POD was highest at 0.65 μmol min−1mg−1 in the 0.1 m3 ·m−3 treatment plot and lowest at 0.05 μmol min−1mg−1 in the 0.3 m3 ·m−3 treatment plot, which was not much different from the 0.4 m3 ·m−3 treatment plot. Likewise, MDA was also highest at 2.50 μmol·g−1FW in the 0.1 m3 ·m−3 treatment plot and 0.65 μmol·g−1FW in the 0.2 m3 ·m−3treatment plot, which was similar to the 0.4 m3 ·m−3 treatment plot. Proline was highest at 333.89 μg·g−1 FW in the 0.1 m3 ·m−3treatment plot, and there was no statistical significance in others such as 0.2, 0.3, and 0.4 m3 ·m−3 treatment plots (Fig. 3).Chlorophyll fluorescence and chlorophyll content
Chlorophyll fluorescence and chlorophyll content
As a result of analyzing the physiological response of Farfugium japonicum (L.) Kitam. according to VWC, chlorophyll fluorescence was highest at 0.81 in Week 10 for the 0.3 m3 ·m−3 treatment plot and at 0.77 in the 0.1 m3 ·m−3 treatment plot, showing a decrease from Week 1. The chlorophyll fluorescence data for the volumetric water content treatment of 0.1 m3 ·m−3 were measured from all individuals that survived, without considering the survival rate. The 0.2 m3 ·m−3 treatment plot did not show a statistical difference from the 0.3 and 0.4 m3 ·m−3 treatment plots. Total chlorophyll content was highest at 5.430 mg·g−1 FW in the 0.3 m3 ·m−3 treatment plot, followed by 4.666 mg·g−1 in the 0.2 m3 ·m−3 treatment plot (Fig. 4).
- Conclusion
- Conclusion
As the volumetric water content increased, the growth of F. japonicum also increased, with highest growth observed in the 0.3 and 0.4 m3 ·m−3 treatment plots, particularly evident in measurements of root length and dry weight. In these parameters, the 0.3 and 0.4 m3 ·m−3 treatment plots exhibited over a 1.5-fold difference compared to the 0.1 m3 ·m−3 treatment plot (Table 1). Water stress is closely related to the water holding capacity of the soil where plants grow, and the EAW of general substrate is known to range from 27 to 37% (pF 1.0 – 1.7), and the WBC from 7 to 10% (pF 1.7 – 2.0) (Choi et al., 2009). In this study, growth was excellent in the 0.3 and 0.4 m3 · m−3 treatment plots (Fig. 5). However, the 0.3 m3 ·m−3 treatment was in the WBC range with low moisture utilization efficiency, and the root length in the 0.2 m3 ·m−3 treatment showed no significant difference from the 0.3 and 0.4 m3· m−3 treatments, and the survival rate was about twice as different compared to the extremely dry 0.1 m3 ·m−3 treatment during the 10-week experiment. Considering this, there seems to be resistance to dryness.Continued water stress is known to bring changes to physiological responses such as osmotic pressure, antioxidant response, and photosynthesis activity in addition to plant growth (Duan et al., 2007; Praba et al., 2009). SOD and POD are antioxidant response elements that are known to increase to reduce active oxygen that appears when plants are stressed (Singh et al. 2015). MDA serves as a biomarker indicating lipid peroxidation, a consequence of oxidative stress (Sofo et al., 2004) and higher MDA concentration implies greater exposure to moisture stress. Proline acts as an osmotic regulator that regulates the water potential of plants (Binzel et al., 1985), and it increases when plants are under water stress (Kwon et al., 2022; Jin et al., 2017). For Farfugium japonicum (L.) Kitam., SOD, POD, and MDA increased in the 0.2 m3 ·m−3 treatment in dry state, but there was not much difference in SOD and MDA from the 0.4 m3 ·m−3treatment plot. Proline did not show statistical significance from the 0.3 and 0.4 m3 · m−3treatment plots showing excellent growth, indicating that the plant can effectively deal with drought stress.When there is environmental stress such as moisture, salt, and light in plants, photosynthesis efficiency may decrease, thereby deteriorating growth (Chaves and Oliveira, 2004). Chlorophyll fluorescence is a non-destructive method that can determine the level of stress in plants (Lee et al., 2021). Among them, Fv/Fm is an index that can examine the maximum level of photosynthesis for a leaf, and the plant is not stressed within the range of 0.78 – 0.84, and extremely stressed when it falls below 0.78 (Yoo et al., 2012; Hazrati et al., 2016; Kalaji et al. 2017). Chlorophyll content can be utilized as an indicator to confirm physiological responses associated with aesthetic value, such as flowering in plants (Seo et al., 2022). For F. japonicum, this began to decrease after Week 1 of the experiment in the 0.1 m3 ·m−3 treatment down to 0.77 in Week 10, proving that the plant is stressed, but there was no statistical significance between the 0.2 – 0.4 m3 ·m−3 treatments. This suggests that F. japonicum possesses sufficient drought tolerance to avoid chlorophyll damage up to the volumetric water content treatment of 0.2 m3 ·m−3.Under the premise of utilizing substrate identical to the horticultural substrate used in this experiment, it was determined that the minimum soil moisture content required to maintain the growth and aesthetic value of F. japonicum in indoor gardens is 0.2 m3 ·m−3. In the case of F. japonicum, the survival rate at the volumetric water content of 0.1 m3 ·m−3 was less than 50%, but at 0.2 m3 ·m3, survival rate was 80%. At the volumetric water content of 0.3 m3 · m−3, the plants maintained their aesthetic value while showing highest amount of growth, and were least impacted by drought stress as indicated by chlorophyll fluorescence, chlorophyll content, and antioxidant enzyme activity. The inherent drought tolerance of F. japonicum could be interpreted as deriving from the environmental conditions of the southern coastal evergreen forests where it inhabits. Broadleaf evergreen trees tend to have high shade tolerance (Hallik et al., 2009), and shade tolerance generally are positively correlated with canopy density (Valladares and Niinemets, 2008). Moreover, broadleaf evergreen trees tend to produce slowly decomposing litter (Zukswert and Prescott, 2017). By interception, high density canopy layer and slowly decomposing litter layer can decrease supply of moisture to the forest floor, inducing the formation of a dry environment (Barbier et al., 2008). In addition, coastal forests, being exposed to salt-laden sea winds, are composed of salt-tolerant species (Woods et al., 2020), and salt tolerance is known to be associated with drought tolerance (Bartels and Sunkar, 2005).If further research on determining the adequate volumetric water content for maintenance of the aesthetic value of garden plants like F. japonicum is conducted across more diverse plant groups and quantitative data are accumulated, it would aid in increasing the efficiency of water resource management for indoor garden management using automated irrigation systems. Furthermore, it could contribute to the establishment of a foundation for automated irrigation management in outdoor gardens.
Fig. 1
Moisture retention curve and range of the easily available water (EAW) and water buffering capacity (WBC) of the substrate.
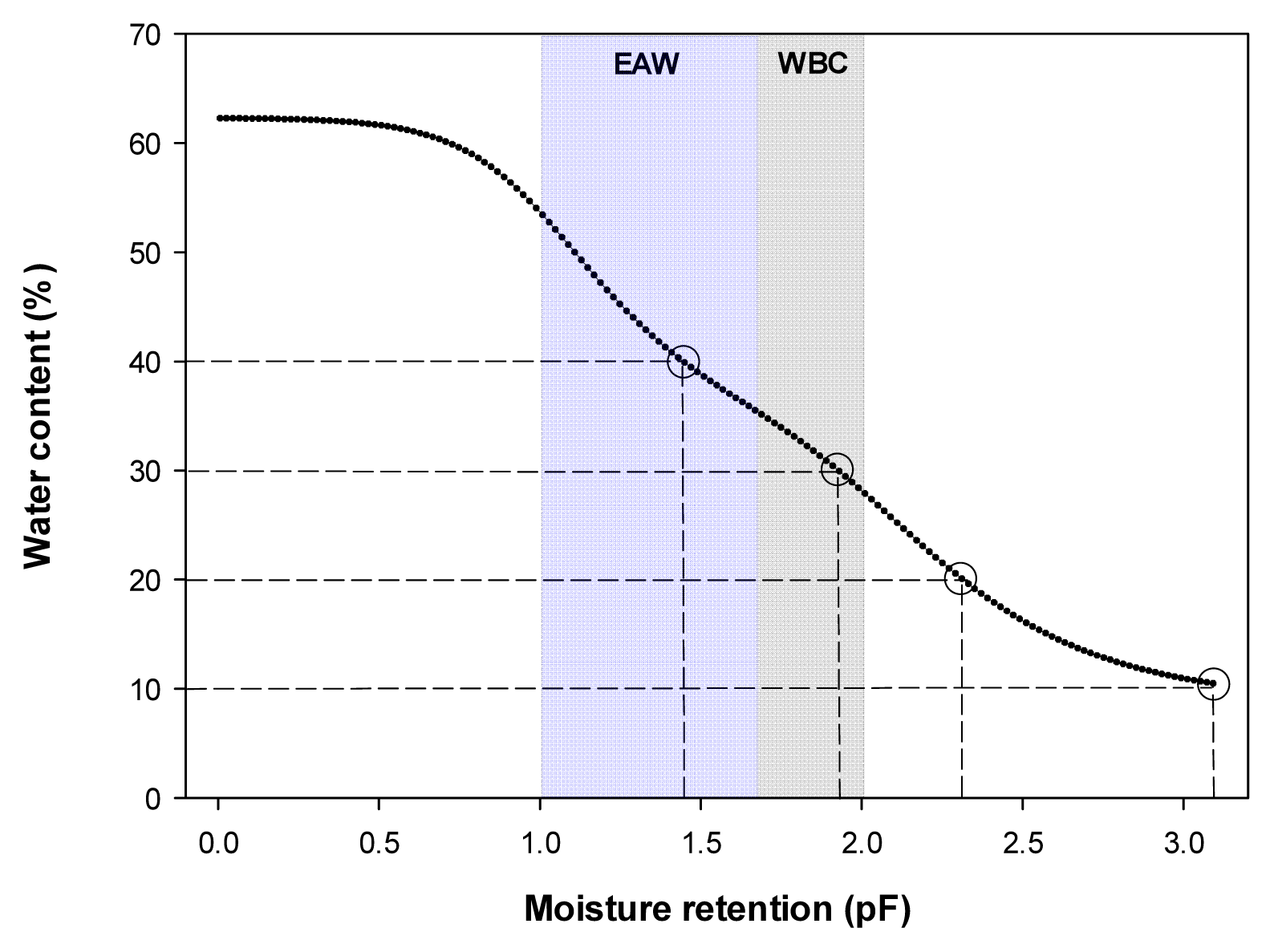
Fig. 2
Average volumetric water content (VWC) of soil as maintained by an automated irrigation system.
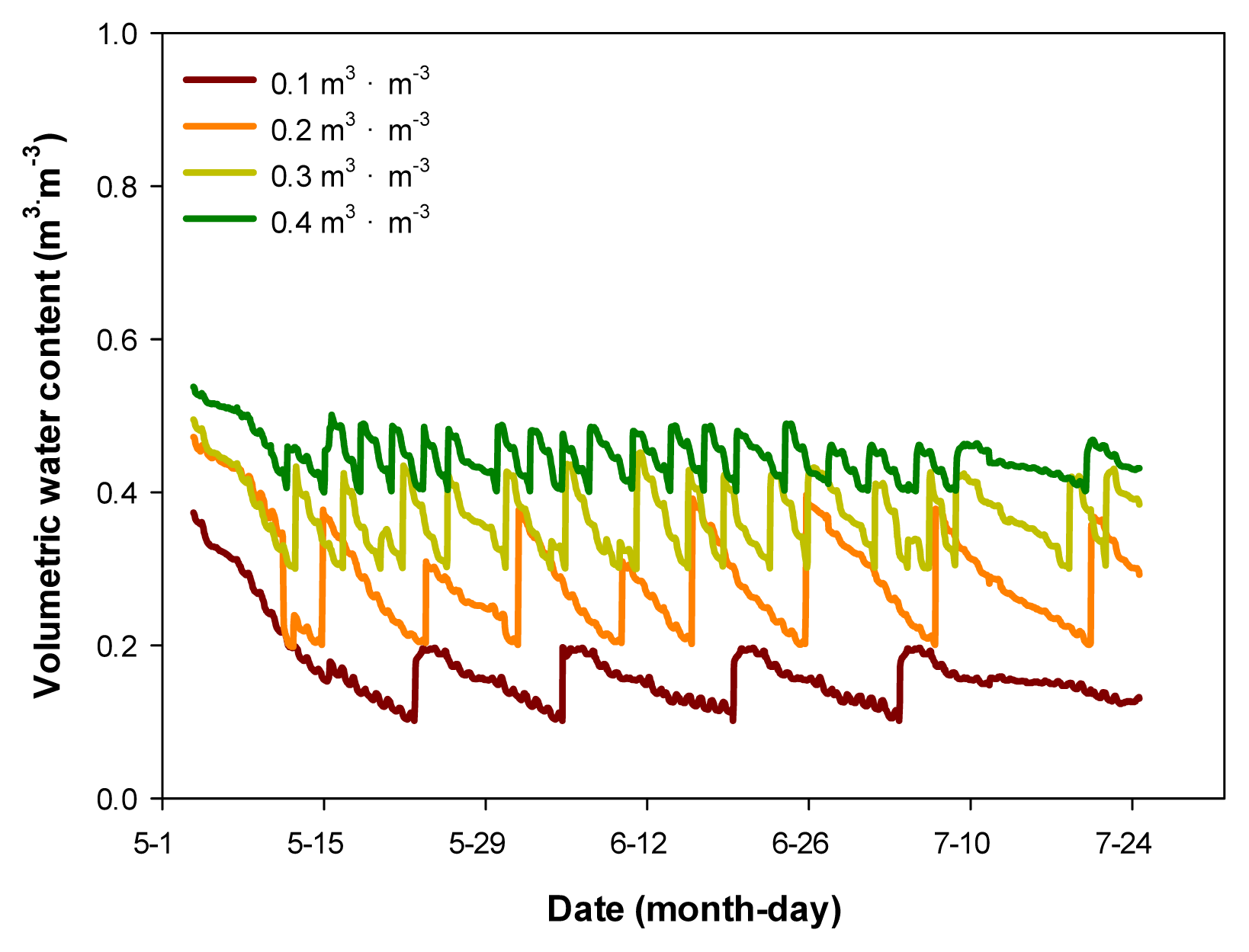
Fig. 3
Effect of soil on superoxide dismutase (A), guaiacol peroxidase (B), malondialdehyde (C) and proline (D) of F. japonicum with different volumetric water contents (θ = 0.1, 0.2, 0.3, 0.4 m3·m−3) at 10 weeks after treatment. Mean separation across the θ treatments followed Duncan’s multiple range test. Error bars indicate SEs ( n = 4 ).
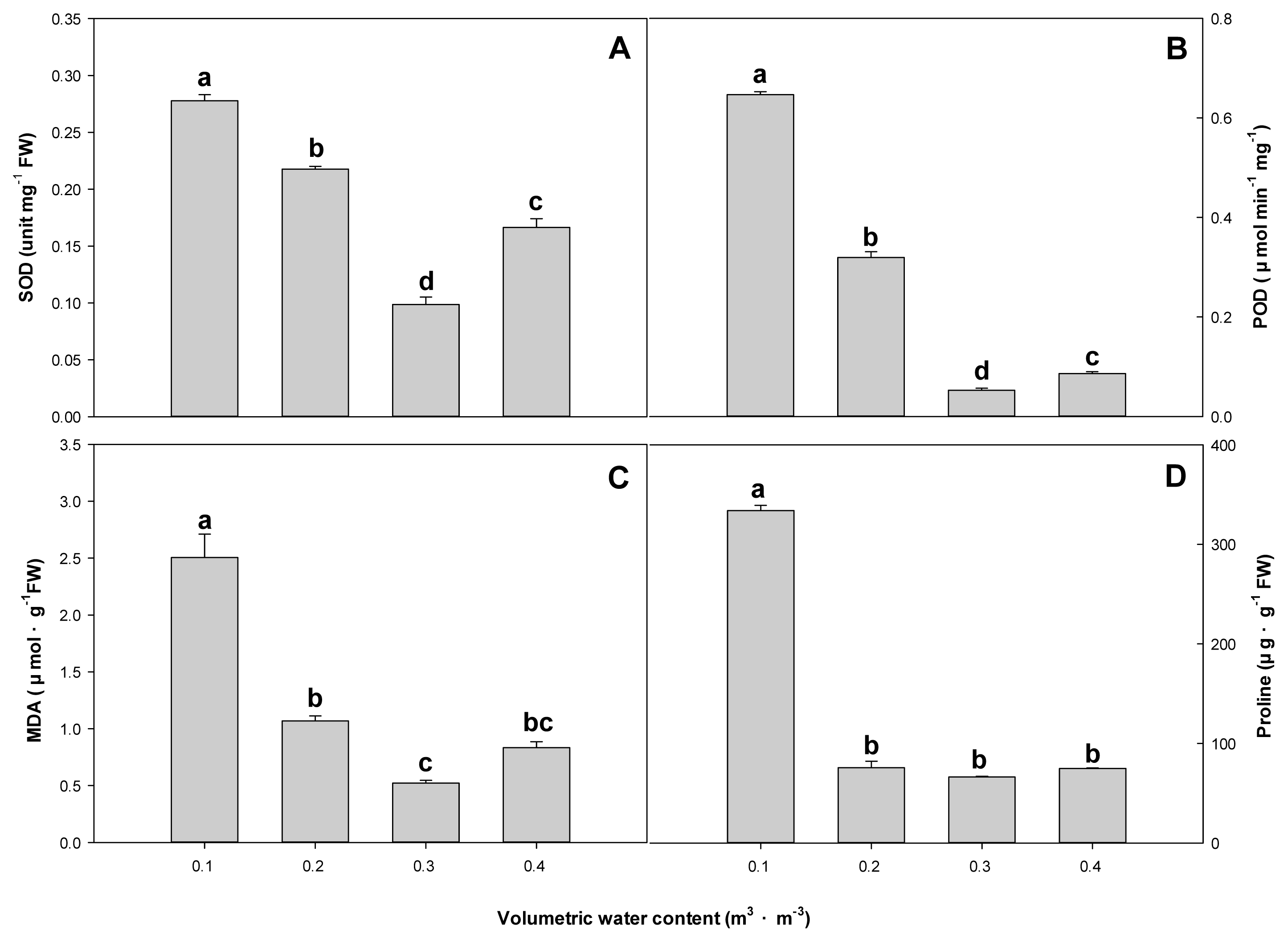
Fig. 4
Maximum quantum yield (Fv/Fm) (A) and total chlorophyll contents (B) of F. japonicum with different volumetric water contents (θ = 0.1, 0.2, 0.3, 0.4 m3·m−3) at 10 weeks after treatment. Mean separation across the θ treatments followed Duncan’s multiple range test. Error bars indicate SEs ( n = 4 ).
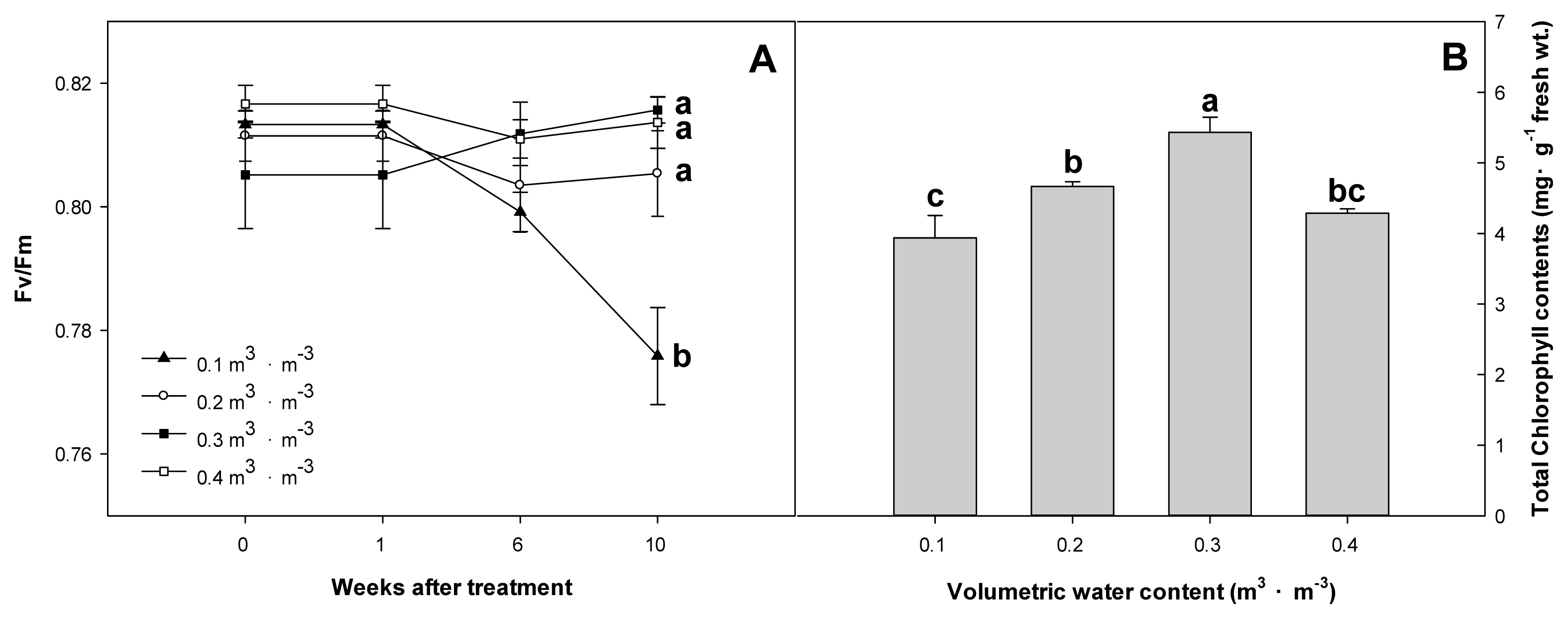
Fig. 5
Picture of F. japonicum plants with different volumetric water contents (θ = 0.1, 0.2, 0.3, 0.4 m3·m−3) at 10 weeks after treatment.
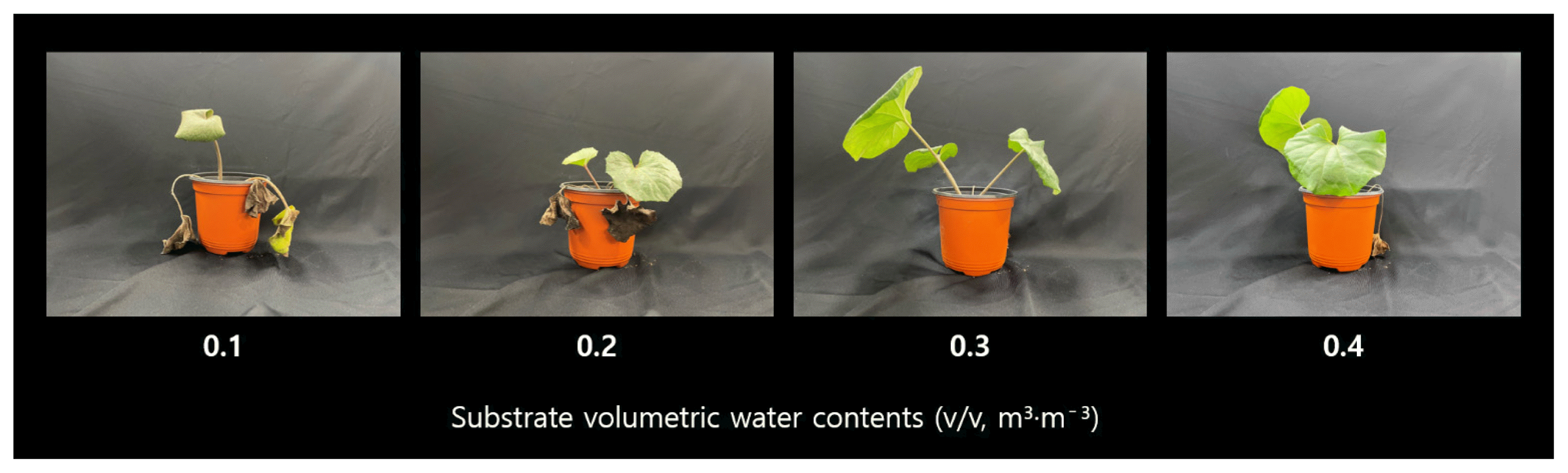
Table 1
Effect of volumetric water content (VWC) on growth characteristics and survival rate of Farfugium japonicum (L.) Kitam. at 10 weeks after treatment
| Volumetric water content (m3·m−3) | Plant height (cm) | Leaf length (cm) | Leaf width (cm) | Leaf number (ea) | Root length (cm) | Fresh weight (g) | Dry weight (g) | Survival Rate (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Shoot | Root | Shoot | Root | |||||||
| 0.1 | 8.0 ± 1.5bX | 6.9 ± 1.2b | 8.4 ± 1.6 | 1.4 ± 0.2b | 7.1 ± 0.9b | 9.6 ± 0.8c | 9.4 ± 0.5c | 0.5 ± 0.1b | 0.7 ± 0.1c | 46.7 |
| 0.2 | 9.6 ± 1.4ab | 8.6 ± 1.1b | 8.5 ± 1.0 | 2.1 ± 0.3ab | 23.0 ± 2.1a | 13.1 ± 1.0b | 13.1 ± 1.0b | 0.8 ± 0.1b | 1.3 ± 0.2b | 80.0 |
| 0.3 | 11.4 ± 0.9ab | 11.2 ± 0.8a | 9.6 ± 0.7 | 2.7 ± 0.2a | 30.1 ± 2.0a | 17.8 ± 1.3a | 17.9 ± 1.0a | 1.5 ± 0.1a | 2.0 ± 0.2a | 100.0 |
| 0.4 | 12.2 ± 1.0a | 12.2 ± 0.9a | 10.4 ± 0.8 | 2.3 ± 0.2a | 25.1 ± 1.3a | 17.6 ± 0.8a | 18.2 ± 1.2a | 1.6 ± 0.1a | 2.1 ± 0.3a | 93.3 |
| Significance | * | *** | NS Y | * | *** | *** | *** | *** | *** | |
- REFERENCES
- REFERENCES
Reference
Arnon, D.I. 1949. Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta Vulgaris . Plant Physiology. 24(1):1-15. https://doi.org/10.1104/pp.24.1.1
[Article] [PubMed] [PMC]Barbier, S., F. Gosselin, P. Balandier. 2008. Influence of tree species on understory vegetation diversity and mechanisms involved-a critical review for temperate and boreal forests. Forest Ecology and Management. 254(1):1-15. https://doi.org/10.1016/j.foreco.2007.09.038
[Article]Bartels, D., R. Sunkar. 2005. Drought and salt tolerance in plants. Critical Reviews in Plant Sciences. 24(1):23-58. https://doi.org/10.1080/07352680590910410
[Article]Bates, L.S., R.A. Waldren, I. Teare. 1973. Rapid determination of free proline for water-stress studies. An International Journal on Plant-Soil Relationships. 39:205-207. https://doi.org/10.1007/BF00018060
[Article]Binzel, M.L., P.M. Hasegawa, A.K. Handa, R.A. Bressan. 1985. Adaptation of tobacco cells to NaCl. Plant Physiology. 79:118-125. https://doi.org/10.1104/pp.79.1.118
[Article] [PubMed] [PMC]Chaves, M., M. Oliveira. 2004. Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. Journal of Experimental Botany. 55(407):2365-2384. https://doi.org/10.1093/jxb/erh269
[Article] [PubMed]Choi, J.M., L.Y. Kim, B.G. Kim. 2009. Soiless Substrates Daejeon, Korea: Hakyesa.Du, Z., W.J. Bramlage. 1992. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. Journal of Agricultural Food Chemistry. 40:1566-1570. https://doi.org/10.1021/jf00021a018
[Article]Duan, B., Y. Yang, Y. Lu, H. Korpelainen, F. Berninger, C. Li. 2007. Interactions between drought stress, ABA and genotypes in Picea asperata . Journal of Experimental Botany. 58:3025-3036. https://doi.org/10.1093/jxb/erm160
[Article] [PubMed]Ferreira, A.B., L. Garcia, L.C. Ming, H. Lütken, B.T. Favero. 2019. Preliminary prospects of northwestern Amazonian shade tolerant species with ornamental potential. In IX International Symposium on New Ornamental Crops. 1288:33-42. https://doi.org/10.17660/ActaHortic.2020.1288.5
[Article]Flora of China. 2008 December 15 Farfugium japonicum (L.) Kitam Missouri Botanical Garden. Retrieved from http://www.efloras.org.Gupta, A.S., R.P. Webb, A.S. Holaday, R.D. Allen. 1993. Overexpression of superoxide dismutase protects plants from oxidative stress (induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants). Plant Physiology. 103:1067-1073. https://doi.org/10.1104/pp.103.4.1067
[Article] [PubMed] [PMC]Hallik, L., Ü Niinemets, I.J. Wright. 2009. Are species shade and drought tolerance reflected in leaf-level structural and functional differentiation in Northern Hemisphere temperate woody flora? New Phytologist. 184(1):257-274. https://doi.org/10.1111/j.1469-8137.2009.02918.x
[Article] [PubMed]Hazrati, S., Z. Tahmasebi-Sarvestani, S.A. Modarres-Sanavy, A. Mokhtassi-Bidgoli, S. Nicola. 2016. Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiology and Biochemistry. 106:141-148. https://doi.org/10.1016/j.plaphy.2016.04.046
[Article] [PubMed]Hiscox, J.D., G.F. lsraelstam. 1978. A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian Journal of Botany. 57(12):1332-1334. https://doi.org/10.1139/b79-163
[Article]Jin, E.J., M.G. Cho, E.J. Bae, J.H. Park, K.S. Lee, M.S. Choi. 2017. Physiological responses to drought stress of seven evergreen hardwood species. Journal of Korean Forest Society. 106(4):397-407. https://doi.org/10.14578/jkfs.2017.106.4.397
[Article]Jung, H.J., W.B. Lee. 2021. A study on the implementation of smart farm environment control system using unity and photon. Journal of the Semiconductor and Display Technology. 20(1):104-107.Jung, S.J., J.S. Song, W.S. Kim, L.D. Woo, H.D. Kim, K.J. Kim, E.H. Yu, J.G. Choi. 2008. Evaluation of selected foliage plants for improvement of indoor humidity. Horticulture, Environment, and Biotechnology. 49(6):439-446.Kalaji, H.M., G. Schansker, M. Brestic, F. Bussotti, A. Calatayud, L. Ferroni, V. Goltsev, L. Guidi, A. Jajoo, P. Li, P. Losciale, V.K. Mishra, A.N. Misra, S.G. Nebauer, S. Pancaldi, C. Penella, M. Pollastrini, K. Suresh, E. Tambussi, M. Yanniccari, M. Zivcak, M.D. Cetner, I.A. Samborska, A. Stirbet, K. Olsovska, K. Kunderlikova, H. Shelonzek, S. Rusinowski, W. Bąba. 2017. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynthesis Research. 132:13-66. https://doi.org/10.1007/s11120-016-0318-y
[Article] [PubMed] [PMC]Kim, J.Y., T.H. Oh, B.J. Kim, S.S. Kim, N.H. Lee, C.G. Hyun. 2008. Chemical composition and anti-inflammatory effects of essential oil from Farfugium japonicum flower. Journal of Oleo Science. 57:623-628. https://doi.org/10.5650/jos.57.623
[Article] [PubMed]Kitaya, Y. 2005. Importance of air movement for promoting gas and heat exchanges between plants and atmosphere under controlled environments. Plant responses to air pollution and global change. 185-193.
[Article]Kwon, H.H., H.J. Oh, Y.H. Kwon, S.H. Yang, S.Y. Kim. 2022. Growth and physiological responses of Pseudolysimachion pusanensis Y. N. Lee to NaCl treatment. Journal of People Plants Environments. 25(2):133-141. https://doi.org/10.11628/ksppe.2022.25.2.133
[Article]Kwon, Y.H., S.Y. Oh, S.H. Yang, S.M. Kong, Y.H. Na, Y.H. Rhie. 2022. The growth of Chrysanthemum morifolium according to the mixing ratio of super-absorbent polymer. Flower Research Journal. 30(4):166-171. https://doi.org/10.11623/frj.2022.30.4.01
[Article]Lee, J.H., R.A.M. Cabahug, N.H. You, S.Y. Nam. 2021. Chlorophyll fluorescence and growth evaluation of ornamental foliage plants in response to light intensity levels under continuous lighting conditions. Flower Research Journal. 29(3):153-164. https://doi.org/10.11623/frj.2021.29.3.05
[Article]Lee, J.S., H.W. Oh. 2002. Using trend of Korean native plants for interior ladnscape in Korea. Flower Research Journal. 10(2):91-96.Mackinney, G. 1941. Absorption of light by chlorophyll solution. Journal of Biological Chemistry. 140(2):315-322. https://doi.org/10.1016/S0021-9258(18)51320-X
[Article]Madzhi, N.K. 2021. Control of plant growth by monitoring soil moisture, temperature and humidity in dry climate. 6th International conference on biotechnology engineering. 1192:012027. https://doi.org/10.1088/1757-899X/1192/1/012027
[Article]Middleton, L. 2001. Shade-tolerant flowering plants: Adaptations and horticultural implications. In XX Inter national Eucarpia Symposium, Section Ornamentals, Strategies for New Ornamentals. 552:95-102. https://doi.org/10.17660/ActaHortic.2001.552.9
[Article]Nam, S.Y., D.H. Lee, J.Y. Kim. 2018. Effect of substrate volumetric water content on performance of Ardisia pusilla grown in indoor conditions. Flower Research Journal. 26(3):124-131. https://doi.org/10.11623/frj.2018.26.3.06
[Article]Nam, S.Y., Y.H. Rhie, J.Y. Kim. 2017. Effect of substrate volumetric water content levels on rooting and growth of Hydrangea cuttings. Flower Research Journal. 25:47-53. https://doi.org/10.11623/frj.2017.25.2.02
[Article]NIHHS. 2006 Floriculture research annual report Korea. Kim, K.J; Retrieved from https://www.nihhs.go.kr/.Poorter, H., J. Bühler, D. van Dusschoten, J. Climent, J.A. Postma. 2012. Pot size matters: a meta-analysis of the effects of rooting volume on plant growth. Functional Plant Biology. 39(11):839-850. https://doi.org/10.1071/FP12049
[Article] [PubMed]Praba, M.L., J.E. Cairns, R.C. Babu, H.R. Lafitte. 2009. Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. Journal of Agronomy and Crop Science. 195:30-46. https://doi.org/10.1111/j.1439-037X.2008.00341.x
[Article]Ramachandran, V., R. Ramalakshmi, S. Srinivasan. 2018;An automated irrigation system for smart agriculture using the Internet of Things. In: 2018 15th International conference on control, automation, robotics and vision (ICARCV); pp 210-215. https://doi.org/10.1109/ICARCV.2018.8581221.
[Article]Rao, M.V., G. Paliyath, D.P. Ormrod. 1996. Ultraviolet-B-and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana . Plant Physiology. 110:125-136. https://doi.org/10.1104/pp.110.1.125
[Article] [PubMed] [PMC]Seo, J.H., S.J. Lee, W.S. Kim. 2022. Flowering response of Rhododendron brachycarpum habituated in different environments Ulleung Island of Korea. Flower Research Journal. 30(2):53-58. https://doi.org/10.11623/frj.2022.30.2.02
[Article]Shehadul, I.M., A. Aryasomayajula, P.R. Selvaganapathy. 2017. A review on macroscale and microscale cell lysis methods. Micromachines. 8(3):83. https://doi.org/10.3390/mi8030083
[Article] [PMC]Singh, M., J. Kumar, S. Singh, V.P. Singh, S.M. Prasad. 2015. Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Reviews in Environmental Science and Bio/Technology. 14:407-426. https://doi.org/10.1007/s11157-015-9372-8
[Article]Sofo, A., B. Dichio, C. Xiloyannis, A. Masia. 2004. Effects of different irradiance levels on some antioxidant enzymes and on malondialdehyde content during rewatering in olive tree. Plant Science. 166:293-302. https://doi.org/10.1016/j.plantsci.2003.09.01
[Article]Song, J.S. 2004. Cultivation and using for garden and potted plant of leopard plant(Farfugium japonicum) native to Korea. Landscape Tree Association. 82(9):20-22.Thongbai, P., T. Kozai, K. Ohyama. 2010. CO2 and air circulation effects on photosynthesis and transpiration of tomato seedlings. Scientia Horticulturae. 126(3):338-344. https://doi.org/10.1016/j.scienta.2010.07.018
[Article]Valladares, F., Ü Niinemets. 2008. Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology, Evolution, and Systematics. 39:237-257. https://doi.org/10.1146/annurev.ecolsys.39.110707.173506
[Article]Wang, H., R. Wang, S.P. Harrison, I.C. Prentice. 2022. Leaf morphological traits as adaptations to multiple climate gradients. Journal of Ecology. 110(6):1344-1355. https://doi.org/10.1111/1365-2745.13873
[Article] [PubMed] [PMC]Woods, N.N., J.L. Swall, J.C. Zinnert. 2020. Soil salinity impacts future community composition of coastal forests. Wetlands. 40:1495-1503. https://doi.org/10.1007/s13157-020-01304-6
[Article]Yoo, S.Y., K.C. Eom, S.H. Park, T.W. Kim. 2012. Possibility of drought stress indexing by chlorophyll fluorescence imaging technique in red pepper (Capsicum annuum L.). Korean Journal of Soil Science and Fertilizer. 45(5):676-682. https://doi.org/10.7745/KJSSF.2012.45.5.676
[Article]Zukswert, J.M., C.E. Prescott. 2017. Relationships among leaf functional traits, litter traits, and mass loss during early phases of leaf litter decomposition in 12 woody plant species. Oecologia. 185:305-316. https://doi.org/10.1007/s00442-017-3951-z
[Article] [PubMed]
