도라지의 생산과 가공 향상에 관한 최신 연구 동향
Current Research Trends on Improvement of Balloon Flower Production and Processing
Article information
Abstract
Balloon flower (BF, Platycodon grandiflorum (Jacq.) A.DC.) is a monotypic species of the bellflower family (Campanulaceae), which is native to Asia. In terms of popular culture of east asian country, BF has been in the limelight as flowers, medicine and food. Platycosides (saponins) from the roots of BF are reported to have a wide range of efficacy and characterized by a structure containing a triterpenoid aglycone and two sugar chains. Saponins from BF are of pharmaceutical significance, and their applications are increasing with evidence of their efficacy. As the cultivation area of BF is continually increased in Korea, research for preventing root rot incidence and nitogen fertilizer application on yield of saponin are reported and also storaging, steam heating and bio-transformational method are developed. There are several biological effects of compounds from BF. saponin from BF has cholesterol lowering effects, neuroprotective activity, cytotoxic effects against cancer cells, and polysaccharide has immuno-boosting effects and pheolic compounds from aerial part of BF has anti-oxidative effects. However, The purchase decision factor of consumers in market is cultivate year of BF and producing country are also important as contents of platycodins in BF. This paper reviews the origin of plants, harvest and post harvest processing methods that maximize the value in terms of efficacy of extracts and platicoside from BF.
I Introduction
Balloon flower (BF, Platycodon grandiflorum (Jacq.) A.DC.) is a monotypic species of the bellflower family (Campanulaceae), which is native to Asia, the BF has been in the limelight as flowers, medicine and food. In Japan, traditionally, it have been loved one of the 'Seven Autumn Flowers', and became a crest (Kamon) of several clans. In China, it is used in traditional Chinese medicine. And in Korea, the root has been used as a popular ingredient in salads and traditional cuisine.
BF is a bushy, clump-forming perennial, to 3 feet tall, but often troubled by floppy stems. Cultivars have been bred for smaller size and compact habits throughout July and August, the tumescent buds burst open to blue, pink, or white starry flowers streaked with prominent veins. In terms of landscape and gardening, especially western country, BF are as easy to grow as daylilies, which are probably the most reliable blue bloomers and added benefit, flowering peaks in mid-to-late summer. Cut them in the bud stage, they make great cut flowers. However, in Asian country, BF root used as a cough suppressant and expectorant for common colds, cough, sore throat in folk medicine. Through recent scientific research, its medicinal effect has been improved such as antiallergy, neuroprotective, anti-inflammatory, anti-obesity Antidiabetic and anti-cancer properties.
Identification of these various pharmacological action causes a change in the production volume of BF. In South Korea, since 2006, the total production of volume of BF has been gradually increased and finally in 2012, it was produced an amount of 2.5 folds as in 2006 did (Fig. 1). In China, the largest exporter of BF, due to the increase of its own domestic demand, export volume for Korea was decreased in around 2010. Meantime, the imported root of BF for the pharmaceutical purpose was 429 ton in 2001 but it has been dramatically decreased to 36 ton in 2011. Instead, the imported volume of dried or fresh one for food was 11,652 ton in 2008 and remained at similar level up to now.
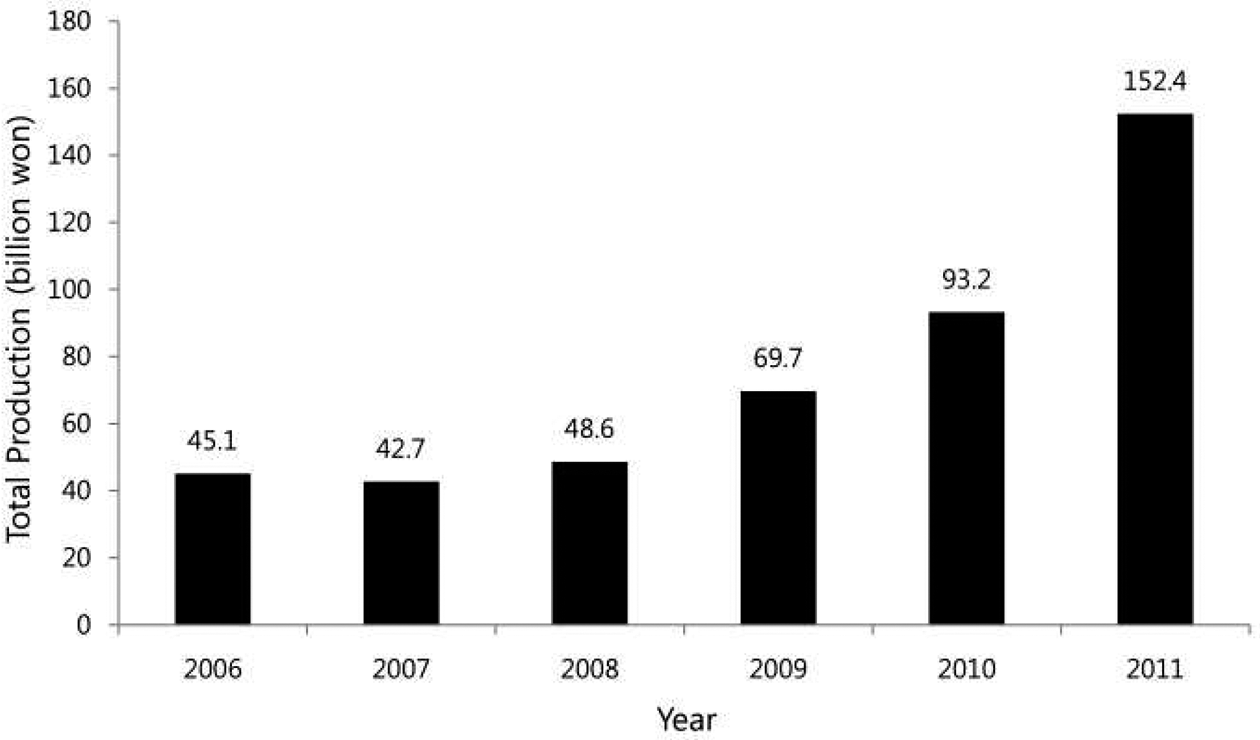
The total production amount of Platycodon grandiflorum’s root from 2006 to 2011 in south Korea (Lee et al. 2014).
Recently, the cultivation area is continually increased in Korea, especially in Gyeoungnam province. Because of consumer's continuous demands and a rising unit price, these trend will be continue for the time being (Lee et al, 2014). The reason of above is, there are sharply increase in the prevalence of chronic respiratory disease and lifestyle-related diseases due to lack of exercise and air pollution. meantime, saponin from root of BF have anti-asthmatic and cholesterol-lowering effect, which is need to take a long period, regardless of the form of food or drugs. Thus, this paper reviews the origin of plants, harvest and post harvest processing methods that maximize the value, in terms of efficacy of Extracts and platicoside from BF.
II Major contents of BF
1 Saponin
Triterpenoid saponins with an oleanene backbone were known as the main chemical constituents of BF. From now on more than 30 kinds of saponin components are founded (Choi et al. 2008b) and 10 major triterpenoidal saponins have been idenfied (Fig. 2). Triterpenoid and steroidal glycosides are referred to collectively as saponins. They have considerable potential as pharmaceutical and/or nutraceutical agents from a variety of natural sources, and have been shown to have hypocholesterolemic, anti-coagulant, anticarcinogenic, hepatoprotective, hypoglycemic, immuno-modulatory, neuroprotective, anti-inflammatory and anti-oxidant activity (Rao and Gurfinkel, 2000). Triterpene saponins with inhibitory activity of glucose absorption may also be used for the prevention and treatment of diabetes. In addition, oleanolic acid glycosides showed other various medicinal effects such as antiallergic, antiinflammatory, antinociceptive, antipruritive, and gastroprotective effects and acceleration of small intestinal transit (Yoshikawa and Matsuda, 2000).

Structure of platycosides. Gen, glucose-glucose; Glc, glucose; Api, apiose; Ac, acetyl (Ha et al. 2006).
2 Non saponin
Phenylpropanoids (PPs) is as a secondary metabolites produced by plants, the molecular basis for the protective action of PPs in plants is their antioxidant and free radical scavenging properties. It has been medicinal use as antioxidant, UV screens, anticancer, anti-virus, anti-inflammatory, wound healing, and antibacterial agents (Korkina, 2007). The phenolic esters found in plants are related to the main functions of suberin and cuticle as a hydrophobic transport barrier against water loss over the plantsurface and as a protectant from pathogens or environmental harm. Their role as a physical barrier, these lipophilic plant surface layers are also important as a protectant from oxidation. Lee et al. (2004b) reported that two PPs isolated BF root are supposed to originate particularly from the suberin of the root have antioxidant activities. Chung et al. (2012) reported that ethyl acetate extracts of BF, fraction of rich phenolic compound, significantly reduced plasma and hepatic lipid levels in the high fat diet induced obse mice, by decreasing oxLDL-induced cell death and lactate dehydrogenase.
Lee et al. (2004a) reported that the petroleum ether fractioned phenolic compound, polyacetylene compound of BF was confirmed to exhibit antioxidant and anticancer activities. Jeong et al. (2010) reported that the butanol fraction of the aerial parts of BF has strong antioxidant activities that are correlated with its phenolic compounds, particularly luteolin-7- O-glucoside and apigenin-7-O-glucoside. Jang et al. (2010) reported that flavonoids and caffeoylquinic acids, isolated from an EtOAc-soluble fraction of the flowers of BF have inhibitory activity on the formation of advanced glycation end products and rat lens aldose reductase in vitro
III Production and Processing of BF
1 Origin of plant and quality evaluation
Gyeongsangnam-do, Korea setting a rules governing preservation and promotion of indigenous balloon flower. Kim et al.(2014) established a technique to differentiate the indigenous balloon flower germ lines with those collected from South Korea and China, to develop RAPD analyses with five different primers exhibited high frequency of polymorphic DNA bands up to 76.9% and phylogenetic tree indicated that some of the indigenous lines can be easily differentiated with others. Park et al. (2005) also analyzed the genetic variation, genetic relationship of BF sample from East-Asia by means of RAPD-PCR markers. Geographically it was divided into 3 different cluster, but inconsistent group also existed, collected from China but they clustered to the same group collected from Korea. Park et al. (2010a) reported that the correlation of character of aerial parts on BF and content of platycodin D(PD). PD was represented highly negative correlation coefficients between plant height, stem diameter, leaf length, root diameter, and root weight. Among the collected resources, PD content of Japanese collections, which is a main medicinal property in BF, was higher than the Chinese and Korea collections. Kim et al. (2007) revealed that Korean BF (esp. 21 years old) contained more deapioplatycoside E, platycoside E, deapioplatycodin D3, platycodin D3, polygalacin D2, platycodin D2 than Chinese BF but had less than half the amount of PD compared to its Chinese counterpart. Because 88% Saponin of Chinese BF is PD, Yan YZ et al. (2012) demonstrated that saponin content of BF is determined by its petal color and geographical location. The accessions with blue flowers dominated over those with white one. especially, PD.
BF with white petal is native to Korea. However, compared to root of BF with white petal, sanpoin from those of blue petal are more distributed in cortex of root (Shin and Kang, 1992).
The purchase decision factor of consumers in market, cultivate year and producing country are also important as contents of platycodins in BF. First of all, identification of the cultivation year of BF is very important in commercial market. Ahn et al. (2011) sugguested the criterion, that is, the characteristics of the cork layer, latex tube, and vessels were shown to be useful keys to confirm the cultivation year of BF. Second of all, Saeki et al. (1999) proposed a reference Platycodins to distinguish BF collected in three Northeast Asia countries. Briefly, BF from Korea, China and Japan has comparative advantage in platydodin C, platycodin D and platycodin A, respectively (Fig. 3). You et al. (2011) determined with 18 platycosides for BF samples cultivated in eight different Korean provinces. Amongst these 18 platycosides, platycoside E (α) showed the highest content, followed by polygalacin D2(β) and 3''-O-acetylplatyconic acid A(γ). The sum of these three compounds was recommended for quality control of balloon flower root for medicinal purposes. And finally they clustered the samples into groups based on platycoside content. Group I, characterized by a high concentration of platycosides, was located near the west coast of Korea, whereas group II, characterized by a low concentration of platycosides, was located inland or in mountainous area(Fig. 4).
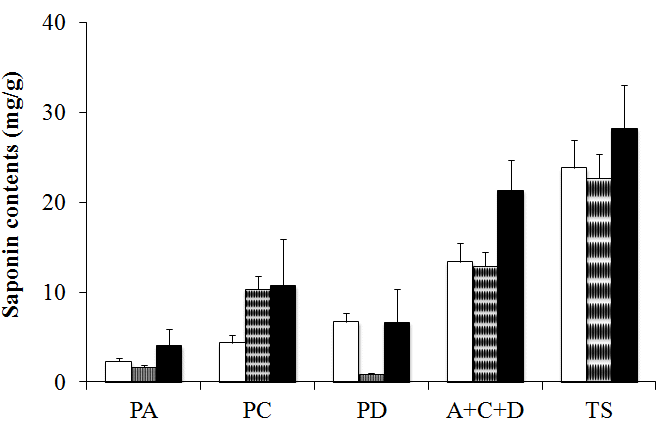
Saponin contents (mg/g) of the BF from different country. □: BF from China, ▣: BF from Korea, ■: BF from Japan, PA:Platycodin A,PC: Platycodin C,PD: Platycodin D, TS: Total saponin (Data from Saeki et. al., 1999, 2003).
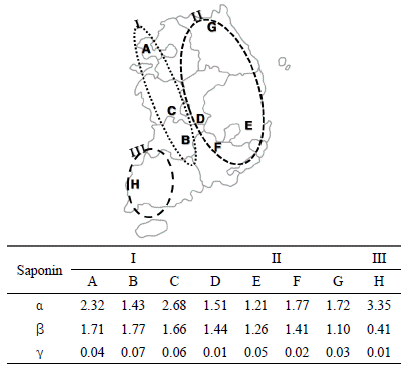
Clustering of the samples based on 3-platycoside content and geographical locations of the cultivated areas (Yoo et. al., 2011).
Triterpenoid saponins with unique chemical features on an oleanene backbone were known as the main chemical constituents of the species and more than 30 kinds of saponin components such as PD have been reported so far (Choi et al. 2008b). Nevertheless, Saeki and Nikaido (2003) suggested quantitative method, that is, Not only for Platycodins, including PA, PC, a PD but for the Polygalacin acids and Platycosides, was a index to evaluate BF for an effective means to the quality evaluation method.
2 Cultivate
Lee et al. (2013a) mentioned that, to optimizing for the pre-seeding treatment on seed germination of BF, germination rate of chilling period 1 week was higher than other treatment. Promptness index(PI) on at 25°C was higher than other temperature. Lee et al. (2000) reported that the data for the management of BF root rot syndrome showed the soil K, NH₄-N content and EC value of injury root were higher than those of normal root. The root CaO content was positively correlated with yield. The Fe and Zn content of normal root were higher than those of injury root, while the T-N, P2O_5 and K₂O content of normal root were lower than those of injury root. The population of bacteria, actinomycetes and bacteria/fungi ratio increased in soil of normal root, but that of fungi decreased. Kim and Cho (2011) reported that correlation on characteristics of Seedling place status and BF root rot incidence. that is, ridge width, soil water content, soil hardness, and cultivation period were positively related with Rhizome rot incidence, however, furrow depth was negatively related with that. So cultivation method should be developed such as making underground ditch or cultivation in well draining soil for escaping excess waster damage (Table 1).

Corelation coefficient between ridge width, furrow depth, and experimental period and Rhizome Rot incidence of Platycodon grandiflorurm (August 10-25, 2010) (Kim and Cho, 2011).
Seong et al. (2004) reported that optimum nitrogen split application ratio of 50:50% improve yield of saponin and quality BF. Lee et al. (2010) reported that Relationship between soil pH and EC, content of exchange calcium in soils for BF cultivation. In brief, regarding chemical properties of soil, content of exchangeable calcium in soils from cultivation BF 3 more consequent years were showed 2~3 time higher level than average upland soil of Korea. Lee et al (1999) reported that the total crude saponin contents were decreased by increasing cultivating years. And they assumed that it because, as Root grows, the relative proportions of tail and cortex of root, which is saponin rich part, were reduced. However, the creating of giant BF by the polyploidy breeding method can maximize its efficacy. Because, in medicinal use, polyploidy may increase the amounts of secondary metabolites. Boo et al. (2013) conducted to obtain tetraploid to have higher contents of pharmaceutical constituents as well as higher yield in BF by colchicine treatment, and their antioxidant activity were compared with diploid. Han et al (2014) shows the way to prevent the occurrence of blue balloon flower in the massive cultivated area of white balloon flower by providing the seedlings raised from in vitro regenerated plants. It is reported that NAA was better than IBA for the induction of root and it took 16.9 days for the induction of rooting on the MS solid media containing 0.5 mg/L of NAA and the final rooting ratio was up to 75%. And also choosing optimized bed soils for the acclimation and growth of in vitro regenerated balloon flower. On 8 weeks after planting of in vitro regenerated plants in pots containing. In the optimized bed soils, the plant hight was increased up to 2-fold , 3.5-fold for the number of leaf and 1.5-fold for the leaf length.
3 Post harvest processing
A variety of techniques that can be influence directly on the content of saponin, such as storaging, blenching and puffing method, have been reported to influence the content of saponin for after harvest. Lee et al (2014a) reported that the high root hardness was significantly related with storing temperature and methods. During 50 days, platycodin D3 and polygalacin of BF which is storaged at room temperature was increased 16-25% than cold storaged one, but it shows a decreasing tendency of after 100 th days. And deapioplatycodin D showed a tendency to increase as the storaging period is longer.
Before convert BF into frozen food materials, blanching process are needed to prevent surface discoloration. Lee et al. (2011c) reported that 90°C for 1 min to be the most highly evaluated in terms of the Storage stability level include hardness, colorness and sensory characteristics. Puffing has induced a porous structure in BF so that the extraction yield increased by enhanced penetration of the extraction solvent into the inner portion. The changes of saponin components in BF by puffing process were reported (Park et al., 2012). Briefly, major changes in appearance were browning and volume expansion, which lead extraction yields and crude saponin yields increase up to 21 % and 41.6% respectively. The DPPH radical scavenging activity of puffed BF also increased three-fold.
Lee et al. (2013b) and Hwang et al. (2011) reported that correlation between heating and antioxidative effects was high. Lee et al. (2013c) assume that high molecular phenolic compounds and phenolic compounds which is bound to the protein was converted to low molecular phenolic compounds by heat treatment. This is due to conjugated phenolic compound of BF was transformed to the free phenolic compound, which is more low molocule, and finally increasing in a total phenolic amount, antioxidant activity increased consequently(Turken et al., 2005). And in addition, by-product of Mailliard reaction, have anti oxidative effect also (Kim et al., 1981). Lee et al. (2013d) reported that steamed BF (Black BF, steam dried BF) showed a higher amount and extraction rate of saponin, almost twice as much crude saponin as BF did.
Ha et al. (2010) have achieved enzymatic conversion of platycosides to PD within 24 hr utilizing cellulase from Trichoderma reesei, that is, cellulase, β-galactosidase, and β -glucosidase are able to transform platycoside E and platycodin D3 into PD. PD was modified with a crude enzyme extract from Aspergillus niger. The modified PD possessed a shorter sugar side-chain, and presented a remarkably reduced cytotoxicity and hemolytic toxicity, whereas Sensory scores for pungency were improved (Wie et al., 2007)
IV Major Efficacy of BF
1 Immune boosting Effects
Park et al. (2010b) reported that BF has an immunogenicity effect as an adjuvant on adaptive immune system. That is, the proliferation of lymphocytes and the antibody titer were increased after BF treatment. The increased subisotypes of antibodies were IgG2 and IgG3 induced from T1-helper cells. Ryu et al. (2014) mentioned water extract from BF enhanced the immune function by regulating the splenocytes proliferation and cytokine production capacity by activating macrophages. Han et al. (2011) reported that enhancing immune system is due to polysaccharide isolated from BF. It’s polysaccharide markedly specific activator of B cells and macrophages but not of T cells. It increase polyclonal IgM antibody production and the proliferation of B cells, and to activate iNOS transcription and NO production in macrophages. Takechi (1995) reported that C-3 of aglycone could affect the haemolytic and adjuvant activities of platycodigenin-type saponins, and that PD had immunological adjuvant activity, and simultaneously elicited a Th1 and Th2 immune response by regulating gene expression of Th1/Th2 cytokines and transcription factors.
Vaccines require association with adjuvants capable of increasing the potency or stimulating the appropriate immune response. Saponins activate immune system, which has led to significant interest in their potential as vaccine. The lead saponin adjuvant is QS-21. However, the high toxicities and undesirable haemolytic effects of QS-21 have been pointed out as the main restriction to its use as adjuvant. Xie et al. (2008) reported that Platycodin D2 could be safely used as adjuvant eliciting Th1 and Th2 immune responses. Haemolytic activity for PD2 than Quil A, which is one of most predminant saponins in Quil A. As adjuvant potential on the cellular and humoral immune responses. PD2 significantly enhanced a specific antibody and cellular response against OVA in mice and modulated the quality of the immune responses resulting in a balanced immunity for broader protection. However, PD2 was showed to be less haemolytic effect than Quil A. Oda et al. (2000) reported that adjuvant activity of saponins does not relate with haemolytic activity.
2 Immune modulating Effects
Kim et al. (2012) reported that fermented BF by Lactobacillus plantarum (LP), up regulate Th1 cytokines, IL-12, p40 and IFN-γ while the levels of the Th2 cytokines IL-4 and 5 were down-regulated. Ha et al. (2014) reported that fermented with Saccharomyces cerevisae decreased the serum levels of Ig E, MDA and reducing Itching reactions in mouse. Oh et al. (2010) demonstrate that BF Ext. inhibits PMA + A23187 induced production of IL-6, PGD(2), LTC(4), β-Hexosaminidase and COX-2 protein. Choi et al. (2009) reported that aqueous extract from the root of BF (21 years old) reduced the OVA-induced upregulation of matrix metalloproteases activity as well as NF-kappaB nuclear translocation. And increased level of the immunosuppressive cytokine IL-10. Reduced thickness of the epidermis/dermis and dermal infiltration of inflammatory cells in the ears. BF also suppressed TNF-α/IFN-γ-induced mRNA expression and production of TARC in HaCaT cells (Choi et al., 2012).
Kim et al. (2010) reported that Only PD significantly suppressed prostaglandin E2 production in rat peritoneal macrophage. Althogh platycodin D3 and oleanolic acid showed no effect at the same concentrations. Oral treatment of BF suppressed AD-like skin lesions according to the assessment of skin severity and epithermal thickness in the DNCB-treated NC/Nga mice. This alleviation was further correlated with a reduction of elevated serum total IgE or cytokine mRNA in the BF-treated group compared (Park et al., 2012).
3 Cholesterol lowering Effects
Unfortunately, drug treatment of obesity despite short-term benefits, is often associated with rebound weight gain after the cessation of drug use, side effects from the medication, and the potential for drug abuse (Abdollahi et al., 2003). It has been reported that BF has an anti-obesity property that prevents mice fed a high-fat diet from becoming obese. BF contains saponin (pladicodins), triterpene (betulin), polysaccharides (inulin) and phytosterols, which may have a beneficial effect on metabolic diseases (Kwon et al., 2009).
The high fat diet caused obesity with fat storage and a reduction in small intestinal sucrase activity. Han et al. (2002) reported that antiobesity action by crude saponins of BF may be due to the inhibition of pancreatic lipase activity and prevent the reduction in small intestinal sucrase activity. Platycodins inhibit the activity of human acyl-Co enzyme A and interfere with the formation of cholesterol micelles (Zhao et al., 2006). Platycodins also competitively inhibit pancreatic lipase (Han et al., 2000; Zhao and Kim, 2004; Xu et al., 2005), considered partly responsible for the reduction in dietary lipid digestion and intake (Zhao et al., 2006). Zhao et al. (2008) reported that Platycodins may play a role enhancing cholesterol excretion thus leading to reductions in plasma and hepatic cholesterol levels. Interestingly anti-obesity effect of platycodin suggests that reductions in body weights due to restricted food intake during the trial. This indicates that Body weight loss effect of BF is via central nervous system inhibitory action(Fig. 5).

Effects of platycodin intake on total cholesterol contents in liver, feces, and red blood cell (A), and fractional synthesis rate (B) (Zhao et al., 2008).
And BF consumption likely increased fecal cholesterol excretion as fecal cholesterol concentration was increased up to 2.5-fold. Cholesterol absorption increased in the animals receiving a high dose of platycodins that is platycodin feeding increased (P < 0.001) cholesterol absorption up to 60%, it is assume that platycodins enhanced intestinal sterol permeability, resulting in increased cholesterol absorption, because saponins have been used to enhance trans-membrane permeability but not cholesterol synthesis. Han et al. (2000) reported that The aqueous extract of BF significantly reduced hepatic triacylglycerol concentrations and inhibit the intestinal absorption of dietary fat by inhibiting its hydrolysis. However Inulin, had no effect on the pancreatic lipase and fatty liver induced by the high fat diet. On the other hand, the total saponin fraction of the aqueous extract inhibited pancreatic lipase activity in vitro. Therefore, the antiobesity effect of the aqueous extract of BF may be due in part to the inhibition of intestinal absorption of dietary fat. Water extract of BF significantly repressed the up-regulation of FABP mRNA expression induced by a high-fat feeding in subcutaneous adipose tissue. Park et al. (2007) reported that water extract of BF inhibited 3T3-L1 pre-adipocyte differentiation and fat accumulation, and also decreased pancreatic lipase activity and plasma TC and TG concentrations. It indicates that BF not only helps to suppress the accumulation of body fat but also helps to degrade accumulated fats. Lee et al. (2012a) reported that white balloon flower (Platycodon grandiflorum for. albiflorum (Honda) H. Hara) extracts ameliorates obesity and insulin resistance in obese mice via the activation of AMPK/ACC pathways and reductions of adipocyte differentiation. Zhao et al. (2005) reported Saponin rich fraction from BF reduce the serum TG and LDL-cholesterol, hepatic TG, the liver surface fat pads and remarkable reduction in calorie intake and however increased fecal TG excretion 2.1-3.2 folds, in dose depending manner.
Anyhow, Obesity’s contribution to type 2 diabetes might be due to dysregulation of adipokines and glucose uptake. Ahn et al. (2012) reported BF extracts on adipokines and glucose uptake. 80% ethanol extract of BF markedly attenuated food intake, body weight, epididymal fat weight, adipocyte size and blood glucose levels by the oral glucose tolerance test in mice, and maintained serum levels of adiponectin, resistin, leptin, fructosamine and triglycerides. Gene expression analysis revealed that PGE up-regulated adiponectin, and down-regulated TNF-α and leptin in fat tissue. In L6 muscle cells in vitro, PGE increased insulin-stimulated glucose uptake.
Hwang et al. (2013) investigate that PD can regulate hepatic lipogenesis via an AMPK-dependent signalling pathway. Lee et al. (2011a) reported that PD inhibits adipogenesis by modulating the WNT/β-catenin pathway, Lee et al. (2012b) reported that molecular mechanism of PD to decrease the expression of adipogenic factors through AMP-activated protein kinase α (AMPKα) in adipocytes and its ability to prevent abdominal fat accumulation. PD significantly reduced fat accumulation by inhibiting adipogenic signal transcriptional factors, consequently, lipid metabolism was improved by increasing AMPKα,reduced PPARγ2 and C/EBPα expression in adipose tissue.
Wu et al. (2012) examined the effects of PD, on human umbilical vein endothelial cells (HUVECs) in vitro. PD increased NO concentration and decreased MDA level induced by oxLDL in the medium of endothelial cells. Moreover, PD significantly inhibited the oxLDL-induced increase in monocyte adhesion to endothelial cells as well as decreasing mRNA expression levels of VCAM-1 and ICAM-1 on these cells. These result shows PD has an anti-atherosclerotic activity.
4 Antidiabetic Effects
Triterpene saponins with inhibitory activity of glucose absorption may also be used for the prevention and treatment of diabetes. Oleanolic acid glycosides were found to have neither insulin-like nor insulin-releasing activity, but they inhibited gastric emptying and glucose-uptake in the small intestine. Investigation of the mode of action revealed that the inhibition of gastric emptying was mediated by capsaicin-sensitive sensory nerves and the central nervous system. Furthermore, oleanolic acid glycosides were suggested to suppress the gastric emptying by stimulating the release and/or production of dopamine to act through dopamine 2 receptors, which in turn causes the release of prostaglandins (Yoshikawa and Matsuda 2000).
Zheng et al. (2007) reported that BF may exert its hypoglycemic action by mechanisms such as inhibition of endogenous glucose production without stimulating insulin secretion (Table 2). Crude saponin of BF alleviated diabetic symptoms and improves glucose homeostasis by enhancing hepatic insulin sensitivity as a consequence of decreasing body fat storage, hepatic insulin resistance and improving insulin signalling, glucose-stimulated insulin secretion from pancreatic β-cells in a mild and non-obese type 2 diabetic animal model, 90% pancreatectomized (Px) diabetic rats, fed a high-fat diet(Kwon et al., 2009). Kwon et al. (2012) reported that Platyconic acid (PA) most effectively increased insulinstimulated glucose uptake in 3T3-L1 adipocytes, possibly in part by working as a peroxisome proliferator-activated receptors (PPAR)-γ activator; however, none of the saponins improved glucose-stimulated insulin secretion in insulinoma cells. PA-treated diabetic mice exhibited the lowest peak serum glucose levels and highest serum insulin levels during the first part of OGTT. PA also improved insulin sensitivity: PA increased glycogen accumulation and decreased triacylglycerol storage in liver, which was associated with enhanced hepatic insulin signaling, while PA potentiated the expression of adiponectin and PPAR-γ in adipose tissue, and improved insulin signaling and increased GLUT4 translocation into the membranes. Table 3

The relative ratio of major platycosides after enzymatic reaction (Ha et al., 2010).
5 Antidemantia Effects
Since the pathology of dematantia is complex, it is too simplistic to assume that antioxidant treatment alone might alleviate or delay cognitive decline in dementia (Williams et al., 2011). Ethanol extract BF improve the cognitive deficit caused by scopolamine and that these effects might be due to BF mediated by inhibition of AChE activity and inhibition of TBARS (Moon et al., 2010). Kim et al. (2004) reported that the total MeOH extract and those with saponin rich fraction ameliorated cogntitive impairment but non-saponin fraction didn't. Choi et al. (2008a) reported that the mice with repeated administration of the root extract of BF, crude saponin fraction and platycoside E, showed a markedly prolonged step-through latency period (STL) on the passive avoidance task performed inducing acute ethanol intoxication in mice. These results suggested that the whole root extract of BF and its saponin platycoside E rather than the PD had an ameliorating effect on the ethanol-induced cognitive dysfunction in mice. Kim and Shin (2013) reported that 80% Ethanol extract of fermented BF with Saccharomyces cerevisae C-2 showed significant anti-amnestic and cognitive-enhancing activities related to the memory processes(Fig. 6).
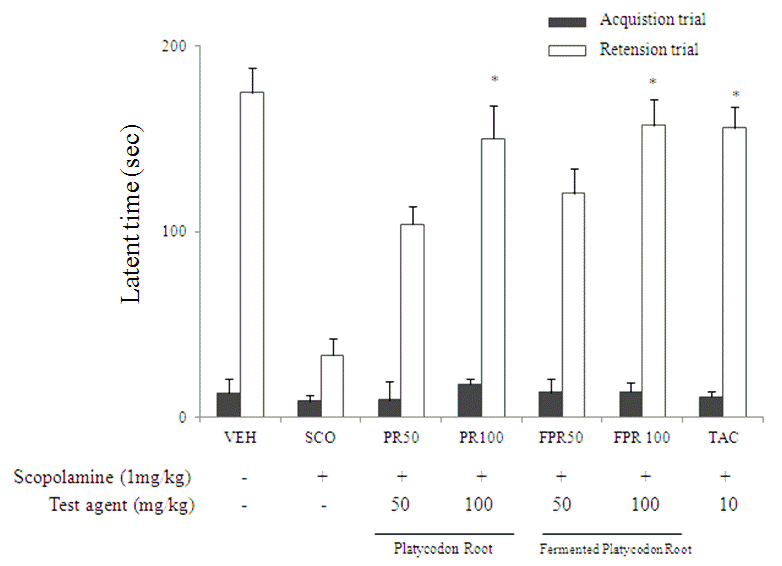
Inhibitory effects of platycodon root (PR), fermented platycodon root (FPR) extract on scopolamine-induced memory impairment in mice in the passive avoidance test. mice were orally administrated PR, FPR 50, 100 ; TAC, tacrine (10㎎/㎏) all values are means ± S.D.(n=6) *Significantly different from the control group (*P<0.05) (Kim et. al., 2013).
6 Anticancer Effects
Due to the great variability of their structures, saponins always display anti-tumorigenic effects through varieties of antitumor pathways (Man et al., 2010). Saponins derived from BF may suppress tumor invasion and migration by inhibiting MMP-2 and MMP-9 activation (Lee et al., 2008). PD can significantly inhibit the proliferation, migration, invasion, or xenograft growth of various human tumor cell lines, including lung, ovary, melanoma, colon (Kim et al., 2005), breast (Chun and Kim, 2013b), liver (Qin et al., 2014), leukemia (Kim et al., 2008; Shin et al., 2009), gastric cancer cells (Chun et al., 2013a) etc. The molecular mechanisms responsible for the anticancer activity of PD involve the suppression of Akt and MAPK pathways (Chun and Kim, 2013; Qin et al., 2014), Its exposure induced apoptosis through caspase-3 dependent PARP, lamin A cleavage induced through Egr-1 activation (Shin et al., 2009). The primary antileukemia activity is induction of endoreduplication and mitotic arrest, as a consequence of suppressing spindle MT dynamics and promoting apoptosis in human leukemia cells (Kim 2008). Furthermore, it has direct cytotoxic effect on human leukemia cells and suppresses telomerase activity through transcriptional and post-translational suppression of hTERT (Dastager et al., 2008). Luan et al. (2014) shows a molecular mechanism of antiangiogenic activity of platycodon root, important hallmark in cancer development. Especially, PD inhibits HUVEC proliferation, motility, migration and tube formation. and it also inhibits the phosphorylation of VEGFR2 and downstream kinases in HUVEC. Chun et al. (2013c) survey on correlation of the chemical structure of BF and anti-proliferative effects on the seven types of cancer cell lines, PD, 2″- O-acetylplatycodin D, 3″-O-acetylplatycodin D, polygalacin D, 2″-O-acetyl polygalacin D, and 3″-O-acetylpolygalacin D, isolated from BF, and prosapogenins which lack the C-3 or C-28 sugar residues, have antiproliferative activity, and the presence of sugar residues, an O-acetyl group on the rhamnose, and a methyl group at C-4 contributes to their cytotoxicity and apoptotic activity.
7 Toxicity
There are two well-known adverse effects of saponins when overdose are hemolytic action and nauseant effect especially platycodin stimulated the vomiting center in anmals, crude platycodin was shown to have an inhibitory action on the central nervous system in the animal in the oral dosage range of 50-200mg/kg (Takagi and Lee, 1972) In humans, there is a potential for some negative effects of excessive intake of saponins, including local effects on gut mucosa and secondary effects produced by mineral interactions decreasing availability for absorption (Milgate and Roberts, 1995).
A dose-dependent effect of PD on the sperm motility and viability was reported (Lu et al., 2013). The maximum spermicidal effect was observed with a 0.25 mM concentration of PD. More than 70% of the PD-treated sperm lost their HOS responsiveness at a concentration of 0.20 mM PD, indicating that PD caused injury to the sperm plasma membrane. PD induced significant damage to both the head and tail membranes of the sperm. And also decreased the fertility to zero in rats, it was non-DNA damaging and not harmful to the vaginal tissue in the rats.
Lee et al. (2011b) reported that Single Oral Dose Toxicity Test of PD. After administration, the slight congestion of lung, atrophy of thymus, cyst in kidney, spleen atrophy or hypertrophy, and hypertrophy and focal hemorrhage of popliteal lymph node detected in the present study as gross findings, and hypertrophy of lung alveolus wall with focal hemorrhages, decreases of lymphoid cells in the cortex of thymus and red pulps of spleen, focal inflammatory cell infiltration in liver, edematous changes on the uterus, and hyperplasia of lymphoid cells in the popliteal lymph node detected as histopathological findings were considered as accidental findings related to the PD treatment. However, approximate LD50 of PD were considered over 2000 mg/kg, and is likely to be safe in humans.
V Discussion
Traditionally BF root is used for cough suppressant and expectorant for cough, sore throat in folk medicine. But current research trend of BF is evaluate its medicinal effect of antiallergy, neuroprotective, anti-inflammatory, anti-obesity, antidiabetic and anti-cancer properties. BF modulate immune system by increase level of the immunosuppressive cytokine and finally improve asthma symptoms, BF not only helps to suppress the accumulation of body fat, but also helps to degrade accumulated fats, and also prevent abdominal fat accumulation. Saponin of BF alleviated diabetic symptoms and improves glucose homeostasis and decreasing body fat storage, hepatic insulin resistance and improving insulin signalling. These result may indicate that lifestyle-related diseases due to lack of exercise. And one thing we should pay attention to BF is it can be administrated a long period, regardless of the form of food or drugs. For this reason, consumer's demand are increased and also they want proof of cultivate year of BF and producing country. In Korean market, there are high demand in BF with white petals, as noted above, Korean native BF have lower contents of PD, but greater diversity on platycodin since saponin component with relatively large polarity is much more than Chinese BF. Saponin with large polarity is need to metabolite to increase bioavailability, and elucidate various efficacy via metablote or heat processing in needed. PD, rich in a BF with blue petal, is mainly focused on cancer research so far, However, with Korean domestic BF, research on cholesterol lowering effect and anti allergic effect is mainly reported in recent year. Judging from the research trend of up to now, more research on memory improvement effects of PD is needed in the future. And besides Root, elucidate efficacy of the flower and aerial parts, rich in a phenolic compounds, are need in the future.
VII 적요
도라지는 세계적으로 1속1종만 알려져 있는 식물로 관상용으로 도 가치가 있는 대표적인 약용 및 식용식물이다. 경상남도에서 제 정된 토종농산물 보존·육성에 관한 조례에 도라지가 포함되는 등 국내에 자생하는 토종 도라지에 관한 관심이 증대되고 있으며 여기 에 최근 국내 도라지 최대 수출국이던 중국의 자국 내 도라지 수요 증가로 수출물량의 감소로 인해 국내의 재배면적 및 생산량이 증가 하고 있는 추세에 있다. 이러한 추세에 발 맞춰 장백도라지의 대량 증식을 위한 조직배양 및 순화 조건, 도라지 재배로 인한 토양의 화 학적 특성변화, 뿌리 썩음 방제, 수확 후 저장 및 가공과정에 관한 연 구결과가 보고되고 있다. 한편, 국내산 토종도라지는 지표물질로 알려진 platycodin D 가 중국산에 비해 현저히 적다는 것이 다수의 연구결과에 의해 입증되고 있다. 하지만 기존 발표된 연구결과를 종합 해 볼 때 도라지의 다양한 약리작용을 platycodin D 가 대표하 지는 않는다는 점이 뒷받침 되므로 국내산 도라지의 고유성분에 대 한 연구와 이를 증가시킬 수 있는 재배방법 및 가공방법에 대한 체 계적인 연구가 시급한 실정이다. 따라서 본 고 에서는 도라지의 기 원, 재배, 가공, 효능에 대해 분야별로 최근 보고된 연구결과를 종합 하여 다음과 같이 국내도라지 연구에 대한 방향을 제안하고자 한 다. 첫째, 외래종에 비해 토종 도라지는 사포닌이 platycodin D에 편중되지 않고 재배기간이 길어질수록, 측근이 발달할수록 극성이 큰 사포닌 생성이 되므로 이러한 사포닌에 대한 생체이용률을 높이 기 위해서는 발효 또는 가열처리에 대한 연구가 필요하다. 둘째, platycodin D가 항암연구에 집중되어 있다면 platycodin D 이외 의 국내산 도라지에 함유된 다양한 사포닌으로 콜레스테롤 감소효 과, 면역조절작용 및 기억력개선작용에 대한 연구가 필요하다. 셋 째, 도라지의 뿌리 이외에 지상부에 함유된 페놀성 화합물의 항산 화작용을 이용한 연구가 필요하다고 결론지을 수 있다.