Effects of Pretreatment of NaOCl, Sucrose, and Benzyladenine on Vase Life and Quality of Cut Flower in Standard Chrysanthemum ‘Jinba’
Article information
Abstract
This study was carried out to examine the effects of NaOCl, sucrose, and BA concentration as pretreatment solution on quality and vase life of cut flowers in Dendranthema grandiflorum ‘Jinba’. Flower diameter, fresh weight, and vase life in control and 0 mg・L-1 NaOCl treatments decreased, and the treatment with 100~200 mg・L-1 NaOCl was more effective in the quality and vase life. In pretreatment with 2.5% sucrose solution, flower diameter and fresh weight decreased and vase life was shortest due to the rapid leaf wilting. However, pretreatment with 0.1% sucrose solution increased the flower diameter and fresh weight, and showed the longest vase life. When more than 80 mg・L-1 BA was treated with pretreatment solution, flower diameter and fresh weight decreased, and vase life was shortened. With pretreatment of 20 mg・L-1 BA, the flower diameter was bigger than in the other treatments, but it was no effect on fresh weight and vase life. Therefore, it was suggested that pretreatment solution mixed with 200 mg・L-1 NaOCl, 0.1% sucrose, and 20 mg・L-1 BA was the most effective for the quality and vase life of cut flowers in standard chrysanthemum ‘Jinba’.
Ⅰ. Introduction
Since cut flowers do not have roots to absorb water and nutrients after harvest, proper nutrients and water should be artificially provided to them. Various cut flower preservatives have been developed to delay the senescence and extend the vase life of cut flowers. And depending on the timing of treatment, they are divided into pretreatment and posttreatment preservatives (holding solution). A pretreatment preservative is a solution which producers use to treat cut flowers after harvest and before shipping, and a holding solution is a solution which wholesalers or retailers, or consumers use while selling or appreciating cut flowers (Son, 1995).
Pretreatment preservatives contain sugar, germicide, ethylene inhibitor, growth regulator, etc. Sugar gives cut flowers energy required for buds to blossom, and also controls osmotic pressure to increase water uptake (Ichimura et al., 1999; Macnish et al., 2010a). Sucrose is widely used as a floral preservative. When 3% sucrose in cut rose and iris ‘Blue Magic’ (Bang et al., 2002; Ichimura et al., 1999; Kim et al., 2005), 10% sucrose in cut sweet pea and Lilium elegans (Ichimura and Hiraya, 1999; Song et al., 1996), and 20% sucrose in Agapanthus orientalis and Iris ‘Discovery’ (Macnish et al., 2010; Mor et al., 1984) were treated, quality and vase life of cut flower were effectively improved.
Germicides are used to inhibit the growth of microorganisms in preservative solutions, and Al2(SO4)3, 8-hydroxyquinoline sulphate (HQS), and 8-hydroxyquinoline citrate (HQC) at 100~ 300 mg・L-1 are widely used (Lee et al., 2011; Mor et al., 1984). Chlorine-based chemicals such as NaOCl and ClO2 (Lee and Kim, 2013; Lee and Kim, 2014; Macnish et al., 2008; Xie et al., 2008) were reported to be effective to preserve cut rose, gerbera, and chrysanthemum.
Cut flowers such as carnation, iris, Cymbidium, and Dendrobium are sensitive to ethylene, and thus senescence is accelerated by ethylene. Ethylene inhibitors such as silver thiosulfate (STS) (Ichmura and Hiraya, 1999; Kim and Lee, 2010) and AgNO3 (Huh et al., 2015; Lee et al., 2011) or ethylene synthesis inhibitors such as aminooxyacetic acid (AOA) (Rattanawisalanon et al., 2003), aminoethoxyvinylglycine (AVG) (Kwack et al., 1996) and 1-methylcyclopropene (1-MCP) (Kim et al., 2010; Lee et al., 2010) can extend the vase life of cut flowers. In addition, gibberellic acid (GA), 6-benzylaminopurine (BA) and thidiazuron can be used to maintain water balance, inhibit wilting and the creation of ethylene, and maintain the turgor pressure of peduncle. They are effective to maintain the freshness of cut flowers such as Lisianthus (Musembi et al., 2015), Gerbera (Danaee et al., 2012), Iris (Macnish et al., 2010a), and Dahlia (Shimizu-Yumoto and Ichimura, 2013).
The quality of cut flowers is determined by the conditions of cut flowers after harvest, including pretreatment, precooling, storage, shipping, etc. When cut chrysanthemum is treated with STS and sucrose before being exported, its vase life is extended by 2 days (Song and Lee, 1999). When cut flowers were stored and shipped at 5°C rather than 20°C or 35°C, their quality was better and their vase life was longer (Suh et al., 2011; Yoo and Roh, 2015a). When chrysanthemum‘Jinba’ was stored at 4°C for 5~10 days, and 1°C for 20 days, their fresh weight, flower diameter and vase life were effectively maintained (Yoo et al., 2014).
In the case of cut chrysanthemum distributed in Korea, their bases are dipped in hot water for 20 seconds as pretreatment in horticultural farms (Lee and Kim, 2002), but hot water dipping is banned when they are exported to Japan. Lee and Lee (2015) found that the vase life of cut Dendranthema grandiflorum ‘Baekma’ was extended when they were treated with NaOCl after harvest, proving the possibility of NaOCl as a pretreatment preservative.
Based on the previously reported studies, this study aimed to identify the effects of NaOCl, sucrose and BA as pretreatment preservatives after harvest and before distribution on the quality and vase life of cut flowers particularly for standard chrysanthemum‘Jinba,’ one of the most widely being grown cultivars in Korea.
Ⅱ. Materials and methods
1. Quality and vase life of cut flower by NaOCl concentrations
The cut flowers used in this experiment were standard chrysanthemum‘Jinba’ in the phase 3 (Yoo and Roh, 2015a) that was harvested on March 30 in a horticultural farm in Haenam-gun, Jeonnam, Korea. The harvested flowers were stored in cardboard boxes and transported for an hour to a laboratory of floriculture in Mokpo National University. They were cut to 80 cm again, and leaves on the stem part up to 30 cm from the base were removed. After that, their flower diameter and fresh weight were measured. To compare the quality of cut flowers depending on different NaOCl concentrations as a pretreatment preservative, the cut flowers were divided into a control group (non-treatment), and several experimental groups which were treated with 0, 50, 100, 200, 400 and 800 mg・L-1 NaOCl for 6 hours. After treatment, their flower diameter and fresh weight were measured again. Square-shaped vases were filled with distilled water (a holding solution), and 5 cut flowers were put in each vase. For each treatment group, the experiment was conducted with 3 replicates. The conditions of a thermostatic chamber were maintained at 25±2°C, 50±5% relative humidity, and 140 μmol・m-2 ・s-1 PPED (light intensity), and their fresh weight, flower diameter and vase life were measured every 2 days based on the standards suggested by Yoo and Roh (2012).
2. Quality and vase life of cut flower by sucrose concentrations
In this experiment, the same flowers used in the previous experiment, but harvested on April 15 were used. The flowers were cut to 80 cm again prior to pre-treatment, and leaves on the stem part up to 30 cm from the base were removed. After that, their flower diameter and fresh weight were measured. Pretreatment solutions were made of 100 mg・L-1 NaOCl with 0, 0.02, 0.1, 0.5 and 2.5% sucrose, and the cut flowers were treated with the solutions for 6 hours. Their fresh weight, flower diameter and vase life after treatment were measured based on the same standards mentioned above.
3. Quality and vase life of cut flower by BA concentrations
In this experiment, cut flowers of standard chrysanthemum ‘Jinba’ that was harvested on April in a horticultural farm (Heaven FC Co., LTD.) located in Jeonju, Jeonbuk, Korea was used. The harvested flowers were stored in cardboard boxes and transported for 2 hours to a laboratory of floriculture in Mokpo National University. They were cut to 80 cm again, and leaves on the stem part up to 30 cm from the base were removed. After that, their flower diameter and fresh weight were measured. Pretreatment solutions were made of NaOCl (200 mg・L-1 ) and sucrose (0.1%) with 0, 10, 20, 40, 80, 160 and 320 mg・L-1 BA, and the cut flowers were treated with the solutions for 6 hours. Their fresh weight, flower diameter and vase life after treatment were measured based on the same standards mentioned above.
4. Statistical analysis
The experiments were arranged in a randomized complete design with 3 replications. Statistical analyses were carried out using the IBM SPSS Statistics for Windows, Version 20.0 software (IBM Corp., USA) and Sigmaplot (Version 12.01, Sistat Software Inc., San Jose, CA, USA). The experimental results were subjected to an analysis of the variance (ANOVA). Duncan’s Multiple Range Test was used to compare means at P ≤S 0.05.
Ⅲ. Results and discussion
1. Quality and vase life of cut flower by NaOCl concentrations
Standard chrysanthemum ‘Jinba’ was harvested and pretreated with different concentrations of NaOCl. There were no changes in the flower diameter of the cut flowers before pretreatment and after 6 hours of pretreatment (Fig. 1A). The pretreated cut flowers were put in holding solutions, and the flower diameter of the pretreated cut flowers significantly increased for the first 8 days, and continued to slowly increase up until the 14th day. Since then, the diameter started to decline slightly. The flower diameter of the control group was small in general, and after 14 days, its diameter was 9.9 cm, the smallest among 7 groups. Among the experimental groups, the flower diameter of those treated with 0 and 50 mg・L-1 NaOCl was smaller than that of other experimental groups. There was no statistical significance between those treated with 100~800 mg・L-1 NaOCl, but the flower diameter of the group treated with 100 mg・L-1 NaOCl was the largest (11.7 cm) on the 20th day, and that of the group treated with 200 mg・L-1 NaOCl was the largest (11.8 cm) on the 14th day.
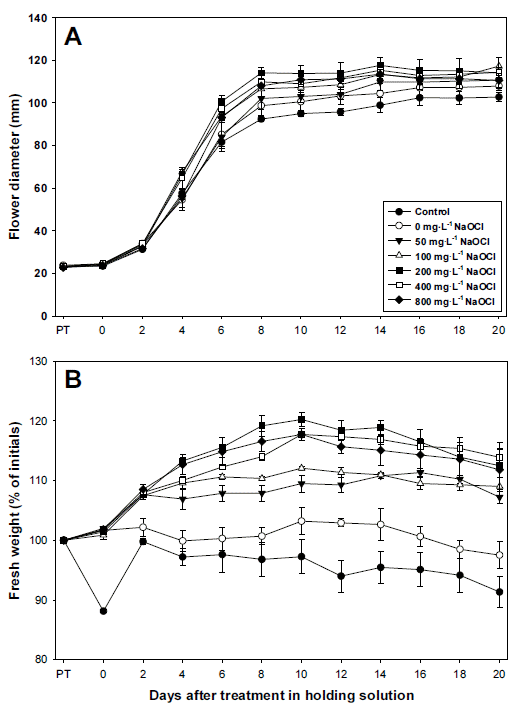
Changes in flower diameter (A) and fresh weight (B) of cut standard chrysanthemum ‘Jinba’ in holding solution after pretreatment with different concentrations of NaOCl. PT: pretreatment time. Vertical bars indicate standard deviation (n=3).
The fresh weight of the control group declined to 88% after the 6-hour pretreatment, and rapidly increased to 100% on the 2nd day in the holding solution (Fig. 1B). However, over time, its fresh weight continued to decline, and was lower than that of other experimental groups. Those pretreated with NaOCl showed no significant change in fresh weight after the 6-hour pretreatment (101~102%), but over time in the holding solution, the fresh weight of the experimental group treated with 0 mg・L-1 NaOCl was the lowest. That of the experimental groups treated with 50 and 100 mg・L-1 NaOCl significantly increased to 107~ 108% in the holding solution until the 2nd day. Since then, there was no significant change observed, and started to decline on the 14th~16th day. The fresh weight of the experimental groups treated with 200~800 mg・L-1 NaOCl was in general higher than other experimental groups, and the weight continued to increase until the 10th day. Since then, it started to decline slightly, but it was still over 112% up until the 20th day. That of the group treated with 200 mg・L-1 NaOCl increased to 120% on the 20th day, higher than other experimental groups.
The vase life of cut flowers was also compared after pretreating experimental groups with different concentrations of NaOCl (Table 1). Those of the control group and the experimental group treated with 0 mg・L-1 NaOCl were 16.1 and 17.0 days respectively, shorter than other experimental groups. However, the vase life of the experimental groups treated with 100~400 mg・L-1 NaOCl was 19.3~19.8 days, 3.2~3.7 days longer than that of the control group. In particular, in the control group and the experimental group treated with 0 mg・L-1 NaOCl, many wilted lower leaves were observed on the 20th day in the holding solution (Fig. 2).

Cut flower quality affected by NaOCl concentration for pretreatment at 22 days in holding solution of cut standard chrysanthemum ‘Jinba’. Arrows indicate the wilting leaves.
NaOCl is widely used as a germicide, and is known to prevent Botrytis cinerea in cut roses (Macnish et al., 2010b). Cut flowers need to be placed in holding solutions after harvest, and germicides must be used to prevent bacteria or fungi from multiplying within the solutions. When cut chrysanthemum ‘Kyoungsubang’ and cut Lilium oriental hybrid ‘Casa Blanca’ were placed in a holding solution made of carbonated water with 40 mg・L-1 NaOCl, their fresh weight and flower diameter were maintained better and their vase life was extended longer than that of untreated ones (Hwang et al., 2009; Lee et al., 1996). It was also found that the vase life of cut gerbera ‘Julia’, ‘Lorca’ and ‘Vilassar’ placed in a holding solution made of 50 mg・L-1 NaOCl was extended by 2.5 days from that of those placed in a holding solution added with aluminium sulfate, used as a germicide (Macnish et al., 2008). When cut Dendranthema grandiflorum ‘Baekma’ was placed in a holding solution added with 50 mg・L-1 NaOCl, its vase life was 6 days longer than that of the control group (Yoo and Roh, 2015b). NaOCl has been reported to be effective as a pretreatment preservative for certain species of cut flowers. The vase life of cut rose ‘Rote Rose’ treated with 100 mg・L-1 NaOCl was extended by 0.9~ 1.8 days compared to that of the control group (Bang et al., 2002). When spray Dendranthema grandiflorum ‘Leopard’ was dipped in 100 mg・L-1 NaOCl solution for 4 hours and shipped at low temperature, the quality of the cut flower was maintained well and flower senescence was delayed (Lee and Lee, 2013). In this study, it was found that pretreating standard chrysanthemum ‘Jinba’ with 100~200 mg・L-1 NaOCl was effective to secure a better quality and a longer vase life similar to other cut flowers.
2. Quality and vase life of cut flower by sucrose concentrations
Cut standard chrysanthemum ‘Jinba’ was pretreated with 100 mg・L-1 NaOCl at and 0, 0.02, 0.1, 0.5 and 2.5% sucrose for 6 hours, and changes in quality were measured as shown in Fig. 3. The flower diameter of all the groups significantly increased in the holding solution up until the 8th day regardless of pretreatment conditions, continued to increase slowly until the 14th day, and since then, started to decline slightly. The flower diameters of the experimental groups treated with 0 and 2.5% sucrose on the 14th day in the holding solution were 10.8 and 10.6 cm respectively, smaller than other experimental groups. However, that of those treated with 0.1 and 0.5% sucrose was 11.7 cm, and they continued to maintain relatively larger diameters (Fig. 3A).
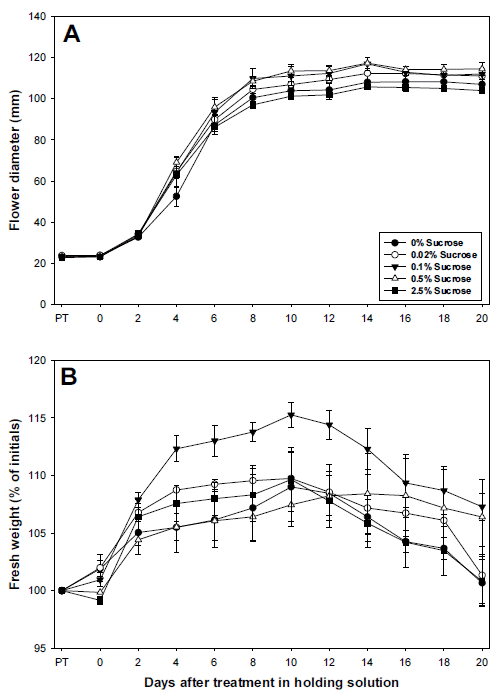
Changes in flower diameter (A) and fresh weight (B) of cut standard chrysanthemum ‘Jinba’in holding solution after pretreatment with different concentrations of sucrose mixed with 100 mg・L-1 NaOCl. PT: pretreatment time. Vertical bars indicate standard deviation (n=3).
The fresh weight of experimental groups after pretreatment and in the holding solution was measured, and that of the experimental group treated with a relatively high concentration of 2.5% sucrose slightly declined to 99% after pretreatment. On the contrary, that of the experimental groups treated with 0, 0.02 and 0.1% sucrose concentrations increased to 101~102%. In the holding solution, the fresh weight of the experimental groups slowly increased up until the 10 th day, and that of the group treated with sucrose at 0.1% increased to 115%, higher than others. Other experimental groups did not show statistical significance (Fig. 3B).
The vase life of the experimental groups treated with different concentrations of sucrose was measured, and that of the group treated with 0.1% sucrose was 20.8 days, the longest among all the experimental groups, and 1.5 days longer than that of the control group (Table 2). The vase life of the group treated with sucrose at 2.5% was the shortest (17.1 days), which is attributable to the earlier wilting of upper and lower leaves than other experimental groups (Fig. 4).
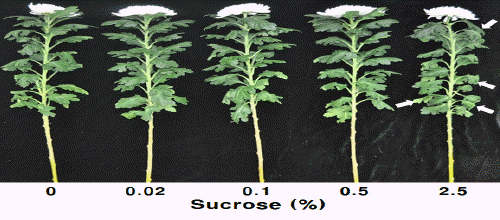
Cut flower quality as affected by sucrose concentration mixed with 100 mg・L-1 NaOCl for pretreatment at 22 days in holding solution of cut standard chrysanthemum ‘Jinba’. Arrows indicate the withered leaves.
When cut flowers such as Platycodon grandiflorus, rose and lily were pretreated with 3% sucrose added with germicides (HQC, HQS and Al2(SO4)3), their fresh weight and solution absorption rate significantly increased, and their flowering rate and vase life effectively improved (Huh et al., 2015; Ichimura et al., 1999; Song et al., 1996). When cut tree peonies were treated with 3% sucrose concentration, the generation of and the sensitivity to ethylene were deterred, and water losses decreased, delaying senescence and extending the vase life of cut flowers (Zhang et al., 2012). When Sandersonia aurantiaca was treated with sucrose at 2%, pectin in cell walls strongly supported the tissues of petals during senescence, improving the quality of cut flowers (O’Donoghue et al., 2002). It was also found that cut sweet pea treated with 0.2mM STS and 10% sucrose for 16 hours showed delayed flowering and extended vase life (Ichimura and Hiraya, 1999). The vase life of cut dutch iris treated with a high concentration of 20% sucrose was extended and their stem growth was accelerated (Macnish et al., 2010a). Likewise, different concentrations of sucrose are used as a pretreatment preservative depending on species of cut flowers. In this study, the leaves of cut standard chrysanthemum ‘Jinba’ treated with 2.5% sucrose wilted early, which seems to be attributable to the inhibited water absorption of the cut flowers due to the high concentration of sucrose. On the contrary, the experimental group treated with 0.1% sucrose showed a larger water absorption rate than other experimental groups, and the diameter and fresh weight of the cut flowers increased. This indicates that pretreating with 100~200 mg・L-1 NaOCl with 0.1% sucrose is effective to improve the quality and vase life of cut standard chrysanthemum‘Jinba’ used in this study.
3. Quality and vase life of cut flowewr by BA concentrations
Cut standard chrysanthemum ‘Jinba’ was pretreated with 200 mg・L-1 NaOCl with 0.1% sucrose and BA different concentrations after harvest, and the quality of the cut flowers was analyzed. There was no significant change in flower diameter before pretreatment, after 6 hours of pretreatment, and until the 2nd day in the holding solution (Fig. 5A). After the 2nd day, however, the flower diameter significantly increased in all the experimental groups up until the 14~16th day, and the group treated with 320 mg・L-1 BA showed the smallest flower diameter (8.5 cm) on the 16th day (Fig. 6). The flower diameter of the group treated with 20 mg・L-1 BA was the largest (11.0 cm), and maintained relatively larger than other groups throughout the experiment period.
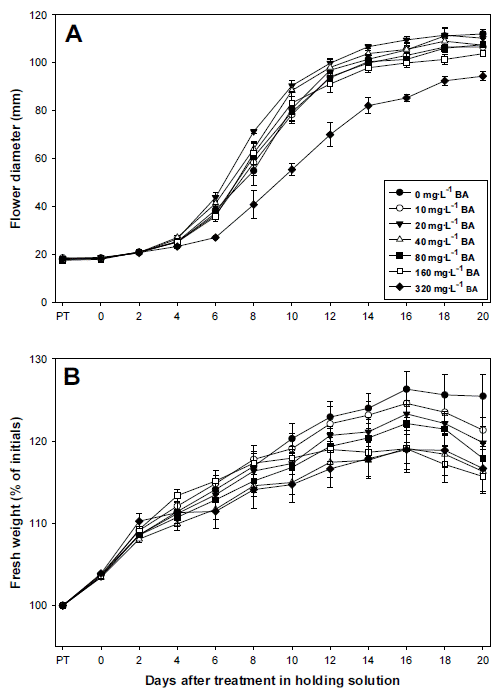
Changes in flower diameter (A) and fresh weight (B) of cut standard chrysanthemum ‘Jinba’in holding solution (distilled water) after pretreatment with different concentrations of sucrose mixed with 200 mg・L-1 NaOCl and 0.1% sucrose. PT: pretreatment time. Vertical bars indicate standard deviation (n=3).

Cut flower quality as affected by pretreatment of BA concentration mixed with 200 mg・L-1 NaOCl and 0.1% sucrose for pretreatment at 22 days in holding solution of cut standard chrysanthemum ‘Jinba’.
There was no significant change in fresh weight among experimental groups after 6 hours of pretreatment, but differences were observed while they were placed in the holding solution (Fig. 5B). The fresh weight of all the experimental groups increased up until the 16th day, and started to decline since then. That of the groups treated with 160 and 320 mg・L-1 BA was 118~119%, relatively lower than other groups. However, the groups treated with 0~20 mg・L-1 BA maintained relatively higher fresh weights after being placed in the holding solution, and they increased to 123~126% on the 16th day.
The vase life of the experimental groups pretreated with different concentrations of BA was measured. That of the group treated with 20 mg・L-1 BA was the longest (21.5 days), but that of groups treated with 0~40 mg・L-1 BA was 20.2~ 21.5, showing no statistical significance. The vase life of groups treated with high concentrations of BA (over 80 mg・L-1 ) decreased as the concentration level increased (Table 3).
BA was found to be effective to inhibit leaf- yellowing in cut chrysanthemum ‘Baekyang’ and ‘Leopard’ as chlorophyll was preserved by dipping them in 25 mg・L-1 BA solution or spraying the solution on leaves (Lee and Lee, 2013; Seo and Kwack, 1994). It was also found that when cut chrysanthemum ‘Reagan White’ was dipped in 10 mg・L-1 BA solution for 24 hours, their chlorophyll efficiency increased and their vase life was extended (Petridou et al., 2001). When cut Lisianthus and Dahlia treated with 15.8 and 50 mg・L-1 BA respectively, the generation of ethylene was inhibited, and water balance and the turgor pressure of peduncle were maintained, delaying the senescence of the cut flowers (Musembi et al., 2015; Shimizu- Yumoto and Ichimura, 2013). In this study, the group of cut standard chrysanthemum‘Jinba’ pretreated with 20 mg・L-1 BA for 6 hours showed the most desirable quality. This indicates that optimal pretreatment conditions (BA concentrations and periods) can differ depending on species of cut flowers. The reason why the flower diameter of the group treated with 20 mg・L-1 BA was the largest seems to be the properly maintained turgor pressure of peduncle and water balance.
The results of this study showed that the most effective way to maintain the optimal quality and vase life of cut standard chrysanthemum ‘Jinba’ was to pretreat them with a solution made of 200 mg・L-1 NaOCl, 0.1% sucrose, and 20 mg・L-1 BA for 6 hours after harvest.


