Anti-obesity effect of Lactobacillus fermented Paeonia japonica, Cnidium officinale, and Angelica gigas nakai
Article information
Abstract
Background and objective
This study evaluated the effects of Paeonia japonica, Cnidium officinale, and Angelica gigas nakai extracts fermented with Lactobacillus in obese mice induced by high-fat diets. Among the fermented liquids, Lactobacillus Paeonia japonica (LBPJ) exhibited high antioxidant activity and a significant total polyphenol content.
Methods
Mice were divided into five groups: normal (NOR), high-fat diet (HFD), high-fat Lactobacillus Paeonia japonica (HLBPJ), high-fat Lactobacillus Cnidium officinale (HLBCO), and high-fat Lactobacillus Angelica gigas nakai (HLBAG). Obesity was induced with a high-fat diet for 8-weeks, and the fermented liquid was administered orally at a dose of 100 mg/kg per day.
Results
The high-fat diet group showed significant weight gain and the group receiving the fermented liquid did not show weight loss. Blood tests showed increased levels of total cholesterol, triglyceride, and high-density lipoprotein (HDL) and decreased levels of low-density lipoprotein (LDL) in the HLBCO group. Creatinine levels remained unchanged, blood urea nitrogen (BUN) levels decreased, and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels increased. As a result of PCR analysis of liver tissue, LBPJ fermented liquid effectively reduced fat accumulation in the liver and inhibited the expression of fat-related genes such as PPARα, PPARγ, CPT-2, and HSL.
Conclusion
These findings suggest that fermented liquids containing Angelica gigas nakai extracts prevent obesity by reducing body fat and blood sugar levels.
Introduction
The incidence of obesity is increasing due to changes in modern people's eating habits, such as increasing intake of high-calorie food and lack of physical activity (Swinburn et al., 2011). Obesity has psychological effects such as depression in humans in addition to cardiovascular diseases such as fatty liver, diabetes, and high blood pressure (Kim et al., 2012). Obesity is a disease in which body fat, not just overweight, accumulates excessively in the body, affecting the production and regulation of active oxygen in the body, increasing the secretion of inflammatory cytokines along with increasing fat tissue (Lempesis et al.; López-Acosta et al., 2023). Obesity promotes the production of free oxygen by cells in the body, and free oxygen (reactive oxygen species, ROS) is naturally produced by cells in the body during oxygen metabolism (Găman et al., 2020). Excessive ROS causes oxidative stress, cell damage, inflammation, and increases the risk of metabolic syndrome-related diseases such as cirrhosis and high blood pressure (Ahmed et al., 2021). Obese people tend to stimulate inflammatory reactions because there are more harmful bacteria than beneficial bacteria in the intestine (Geng et al., 2022). Lactobacillus, a beneficial bacteria in the human intestine, helps prevent vascular diseases, suppress the growth of harmful bacteria, strengthen immunity, and maintain intestinal and liver health (Song et al, 2021; Million et al, 2012). Lactobacillus sp. (L.acidophilus, L.casei, L.plantarum, Bifidus, and S.thermophilus) strains were used in this study. L.acidophilus is found in the mouth, intestines, and vagina, L.casei helps the normal function of the large intestine and digestion, and L.plantarum is found in fermented foods and the human intestine and has an immune effect (Anjum et a l.,2014; Zhu et al., 2021; De Vries et al., 2006). Bifidus limits the growth of some pathogens, including E. coli and staphylococci, by lowering the intestinal pH, and S.thermophilus is highly lactate-producing and antibiotic sensitive (Solís and Gueimonde, 2023; Iyer et al., 2010). Fermentation is a metabolic process in which microorganisms such as yeast and bacteria convert carbohydrates into simple compounds such as alcohol, acid, and gas, and through fermentation, sugars in the intestine are broken down and various beneficial compounds are produced (Du et al., 2011; Kang et al., 2022). Through this process, it improves the taste and aroma of food and helps the body absorb digestion (Oleksy and Klewicka, 2018). Recently, interest in natural products that are safe and have few side effects is increasing, and herbal medicines are attracting attention because they are made of plant-based raw materials with a long history and tradition (Boullata and Nace, 2000; Moreira et al., 2014). The medicinal plants used in this study, Paeonia japonica, Cnidium officinale, and Angelica gigas nakai, have long been used in traditional East Asian medicine as herbs to improve blood circulation (Kim et al., 2014; Kwon et al., 2019). Paeonia japonica (PJ) is a perennial plant belonging to the Apiaceae family that is found in Korea, Japan, China, and Sakhalin, and has a bitter and sour taste, which is beneficial to women's health and relieves pain by enriching blood, dissolving blood clots, and improving blood circulation(Ko et al., 2016; Lee et al., 2021). Cnidium officinale (CO) is a perennial plant belonging to the Apiaceae family that is spicy and warm, and removes blood clots to improve blood circulation and remove various inflammations and waste accumulated in the body(Bae et al., 2011; Ma et al, 2023). Angelica gigas nakai (AG) is a perennial plant belonging to the Apiaceae family, mainly found in Korea, Japan, and China, and has sweet, bitter and warm properties (Park et al., 2019; Zhang et al., 2012). Angelica gigas nakai dilates blood vessels and increases blood flow, which helps improve blood circulation, lowers blood pressure and cholesterol, improves cardiovascular health, and also has a pain relieving effect (Lee et al., 2023; Wang et al., 2016). Paeonia japonica, Cnidium officinale, and Angelica gigas nakai used in this study are traditionally widely used herbal medicines, all used to alleviate and improve various blood-related symptoms in the body. To increase the efficacy of this herbal medicine, each extract was fermented using Lactobacillus to produce a fermented liquid containing newly created substances. Lactobacillus strains are generally the most used, have no adverse effects, and are already used to prepare natural fermentation liquids under development for human consumption. The study predicted that the results of the study using fermented liquids would be more effective and milder than conventional oriental extracts, and that the active ingredients of fermented liquids would be easier to digest and absorb in the body, thereby improving treatment promotion effects. In this study aimed to determine whether Lactobacillus fermented liquids containing extracts of Paeonia japonica, Cnidium officinale, and Angelica gigas nakai contain novel functions for obesity control and to investigate whether Lactobacillus have an affect on body fat reduction.
Research Methods
Materials
Paeonia japonica, Cnidium officinale, and Angelica gigas nakai used in this experiment were purchased from Gyeongdong market, and the Lactobacillus was purchased from the Korea Center for Microbial Resources (Korean Collection for Type Cultures, Jeongup, Korea). MRS liquid medium for fermentation (Difco, Msryland, USA) was used, and the experimental diet was a high-fat diet (HFD, 60 energy% fat; Research Diets, NB, USA). ELISA was measured using a mouse TNF-α ELISA kit and mouse IL-6 ELISA kit (AbFrontier, Korea). For RT-PCR (Reverse transcription-polymerase chain reaction) measurement, TRIzol reagent (Sigma Chemical, USA), RT-PCR premix kit and cytokine primer (Bioneer, Korea) were used. The instruments used in the experiment were a shaking incubator (VS-8480SF; Vision Scientific Co., Korea), a rotary evaporator (EYELA, N-1300, Japan), a freeze dryer (Il Shin Bio, Korea), and an auto amino acid analyzer (Hitachi AAA L-8900, Hitachi High-Technologies Co., Tokyo, Japan), ELISA reader (Thermo Scientific, USA), fluoro box (Neo Science, KOR), UV spectrophotometer (Thermo Scientific Multiskan Go UV/Vis, Korea), biochemistry analyzer (Shinyang chemistry, BA400, Korea).
Preparation of fermented liquid LBPJ, LBCO and LBAG from extracts of Paeonia japonica, Cnidium officinale and Angelica gigas nakai
Paeonia japonica, Cnidium officinale, and Angelica gigas nakai were each added to 1kg of distilled water and extracted three times over 24 hours. The extracted three types of Paeonia japonica, Cnidium officinale, and Angelica gigas nakai were concentrated to 37°C using a rotary evaporator and then freeze-drying using a large-capacity freeze dryer to obtain each extract PJ, CO, and AG. Three MRS medium were prepared, and each Lactobacillus was dispensed and incubated at 37°C for 24 hours. The three types of Paeonia japonica, Cnidium officinale, and Angelica gigas nakai extracts obtained in the previous extraction experiment were added to each of the three prepared Lactobacillus liquids by 1% and then placed in a shaking incubator and fermented at 37°C for 3–5 days to obtain the fermented liquids. Afterwards, each fermented liquid was concentrated to 37°C using a rotary evaporator and then freeze-dried using a large-capacity freeze dryer to obtain fermented liquids LBPJ, LBCO, and LBAG (Table 1).
Free amino acid analysis
To analyze free amino acid, 1g of each fermented liquid was taken, 10mL of 80% ethanol was added, and sonicated for 20 minutes, followed by centrifugation at 3,000 rpm and 4°C for 10 minutes. After repeating the above process twice, only the supernatant was collected and filtered with filter paper, dissolved in 1mL of free amino acid sample dilution buffer (pH 2.2), and filtered twice with a 0.45μm nylon syringe filter. After the pretreatment process, the free amino acid content of the test solution was analyzed using an automatic amino acid analyzer. Column flow 1mL/min, injection volume 20μL, wavelength 570nm and 440nm, N2 gas automatic purge was analyzed.
Antioxidant test
The antioxidant experiment measured DPPH radical scavenging activity and total polyphenol content. For DPPH radical scavenging activity, LBPJ, LBCO, and LBAG fermented liquid was prepared at a concentration of 0.157–5 mg/mL using 70% ethanol, and 0.2mM DPPH (2,2-diphenyl-1-picklehydrazyl) was prepared. 100 μL of fermented liquid and 100 μL of the prepared 0.2mM DPPH solution were placed in a 96-well plate, blocked from light, and reacted at room temperature for 30 minutes. This process was repeated three times, and the absorbance was measured at 492 nm using a microplate reader. Ethanol was added instead of the sample as a negative control, and L-ascorbic acid, known to have high antioxidant activity, was used as a positive control, and the IC50 value was calculated using the equation. The total polyphenol content of the fermented liquid was measured according to the Paulin-Denis method. 200 μL of a fermented liquid prepared at a concentration of 10 mg/mL was mixed with 1 mL of 0.2N Folin-Ciacalteu' reagent, reacted for 3 minutes, 800 μL of a 7.5% Na2CO3 solution was added to block light, react at room temperature for 2 hours, and gallic acid was used as a standard reagent.
Abssample : sample absorbance
Absblank : color control absorbance
Abscontrol : negative control absorbance
Animal
4-week-old male C57BL/6 mice were supplied from Central Lab Animal Inc., Ltd. and used in the experiment. The environment of the animal breeding room in this laboratory was maintained at a temperature of 22 ± 2 °C and a relative humidity of 50 ± 10 °C, and a 12-hour day/night cycle was maintained. Mice were provided with sterilized bedding, drinking water, and solid feed freely. Animal experiments were performed with approval from the Animal Experiment Research Ethics Committee of Kunsan National University (approval number: 2021–03). After the mice were fed a regular diet for one week and went through an adaptation period, their body weight was measured and they were randomly divided into 5 groups of 6 mice each. Mice consisted of the normal group (NOR) that consumed the normal diet, the high fat group (HFD) that consumed only the high-fat diet, the Lactobacillus Paeonia japonica (HLBPJ) administration group, the Lactobacillus Cnidium officinale (HLBCO) administration group, and the Lactobacillus Angelica gigas nakai (HLBAG) administration group (Table 2). Excluding the NOR group, the HFD group and the fermented liquids LBPJ, LBCO, and LBAG group were fed a high-fat diet for 8 weeks, and each fermented liquid was orally administered to mice once a day for 8 weeks. During the experimental period, the body weight of the mice was measured once a day at specific times. Mice were fasted for more than 12 hours, sacrificed, anesthetized using CO2, and blood was collected from the heart using a syringe through an abdominal incision. The collected blood was left at room temperature for 30 minutes and then centrifuged at 3,000 rpm at 4°C for 20 minutes using a centrifuge to obtain plasma. After blood collection, the liver, colon, and fat tissue were removed, washed with physiological saline, and weighed.
Biochemistry analyze
Total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, and blood urea nitrogen (BUN) in mice plasma were measured using an automatic biochemical analyzer.
Enzyme-linked immunosorbent assay (ELISA)
In this study, The liver tissue from 6 mice per group was removed and used, 1 mL of 1×phosphate-bufered saline (PBS) was added, crushed, and centrifuged at 7000 rpm for 10 minutes, and then the supernatant was taken. The amount of infammatory cytokine TNF-α and IL-6 was measured using an enzyme-linked immunosorbent assay (ELISA) kit. After adding 100 μL of antigen to the antibody-coated plate, incubated at 37°C for 2 hours, and washed 3 times using a washing buffer. After the addition of 100 μL of the secondary antibody, it was incubated at 37°C for 1 hours and washed 3 times. After adding 100 μL of the TMB solution, incubate at 37°C for 30 minutes, and reacted at room temperature for 10 minutes after adding 100 μL of stop solution, absorbance was measured at 450 nm.
Reverse transcription-polymerase chain reaction (RT-PCR)
RNA was extracted from mice liver tissue using Trizol reagent, and the amount of cytokine mRNA was measured. After adding 500ng of quantified total RNA and 20pmol of positive region primer to the extracted RNA, a total of 20μL of reaction solution was prepared, and the reaction was performed using an RT-PCR premix kit. The cDNA was synthesized by repeating denaturation 35 cycles at 94°C for 40 seconds, annealing at 58–62°C for 40 seconds, extension at 72°C for 90 seconds at 50°C for 30 minutes, and at 94°C for 5 minutes. The obtained PCR product was identified as 1.2% agarose gel electrophoresis, and was observed under a UV lamp. β-actin was used as a control material. The basic sequences of the primers used in this experiment are as follows (Table 2).
Statistical analysis
The experimental results were statistically processed using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA) and expressed as mean ± standard error (mean ± SE) through one-way analysis of variance (ANOVA) and test. In the Duncan's multiple range test, there was a statistically significant difference, and in the case of P < 0.05, it was judged to be statistically significant.
Results and Discussion
Free amino acid analysis
The total free amino acid contents of LBPJ, LBCO, and LBAG were 2962.6 mg, 1964.1 mg, and 2976.4 mg per 100 g dry weight, respectively. Among free amino acids, phosphoserine is an ingredient that supplies nutrients to brain nerve cells and aids cell regeneration (Ma et al., 2011; Ma et al., 2022). In this experiment, LBPJ was the highest at 2144.3 mg/L, LBCO was 337 mg/L, and LBAG was the highest at 2401 mg/L. Glutamic acid was 297.1 mg/L for LBPJ, 241.7 mg/L for LBCO, and 245.4 mg/L for LBAG. It is produced by protein breakdown, but some of it is produced through microbial metabolism to increase immunity and prevent aging (Jara et al., 2021; Kim et al., 2021). Aspartic acid was LBPJ 118.2 mg/L, LBCO 91.3 mg/L, and LBAG 118.2 mg/L, which are used in various biochemical reactions in the body to prevent toxins and neurological diseases (Li et al., 2007; Zhu et al., 2023). In this study, phosphoserine, glutamic acid, and aspartic acid increased significantly, and these amino acid components are effective in preventing obesity by controlling cholesterol levels in the body (Table 3).
Antioxidant test
As a result of measuring DPPH radical elimination activity, the IC50 values of the fermented liquids were LBPJ 2.63 μg/mL, LBCO 3.48 μg/mL, and LBAG 3.58 μg/mL (Fig. 1A). The total polyphenol content was measured at 0.13 mg/g of LBPJ, 0.11 mg/g of LBCO, and 0.1 mg/g of LBAG. Free radicals attack normal cells in the body and act as a cause of aging or various diseases, and antioxidants remove free oxygen toxicity in cells (Moskovitz et al., 2002; Valko et al., 2007). As a result of the antioxidant experiment in this study, LBPJ had high antioxidant activity and total polyphenol content, but overall, the fermented liquid had low antioxidant effect (Fig. 1B) (Fang et al., 2002).
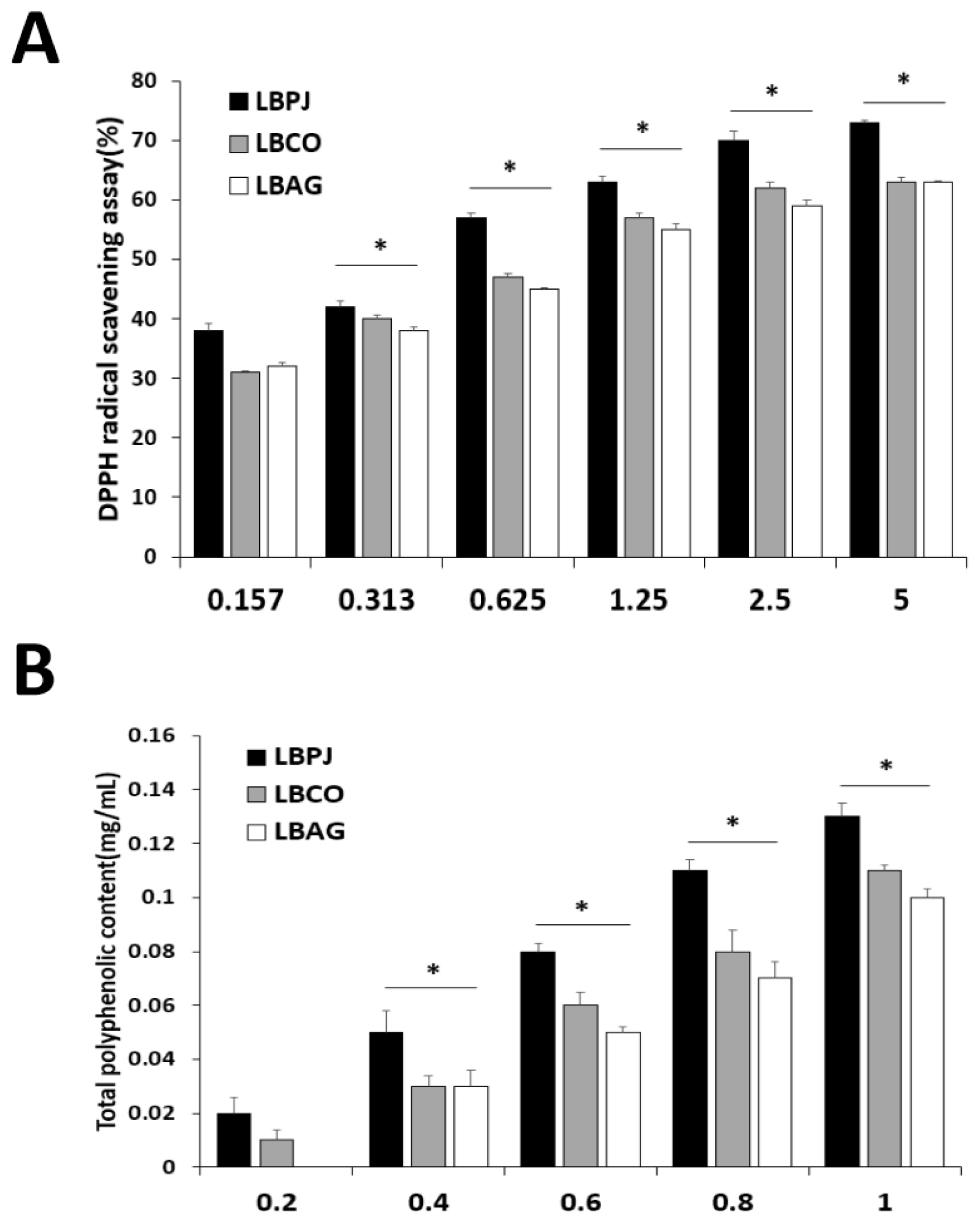
Antioxidant activity of LBPJ, LBCO and LBAG. (A)The DPPH radical scavenging activity of the extracts of LBPJ, LBCO, and LBAG, (B)Total polyphenolic content of extracts of LBPJ, LBCO, and LBAG. LBPJ:Lactobacillus Paeonia japonica, LBCO:Lactobacillus Cnidium officinale, LBAG:Lactobacillus Angelica gigas nakai.
Measurement of mice body weight and liver, colon and adipose tissue weights
If you consume high-fat foods for a long period of time, excessive fat can accumulate in the body and cause chronic inflammation. In this study, mice were fed a high-fat diet for 8 weeks, and a decrease in body fat was confirmed by administering fermented liquid. Before obesity was induced by feeding a high-fat diet, there was no significant difference in body weight between groups, but mice fed a high-fat diet for 8 weeks gained significantly more weight than mice fed a regular diet. During the 8-week experiment, mice weight increased significantly in 47 (± 0.85)g of HFD group compared to 26 (± 0.6)g of NOR group, with no weight loss in 45 (± 0.4)g of HLBPJ group, 42 (± 0.47)g of HLBCO group, and 43 (± 0.5)g of LBAG group (Fig. 2A). The liver, colon, and fat tissue were removed and weighed. The liver weight was 1.9 (± 0.03) g in the HLBPJ group, 0.8 (± 0.03) g in the HLBCO group, and 1.8 (± 0.07) g in the HLBAG group. Compared to 2.0 (± 0.01) g in the HFD group, it significantly decreased in the HLBCO group and increased in the HLBPJ and HLBAG groups (Fig. 2B). The liver weight in the high-fat diet group was significantly increased compared to the general diet group, suggesting the possibility of developing fatty liver disease by accumulating fat in the liver (Lee et al., 2023; Sun and Karin, 2012). Adipose tissue plays an important role in energy storage and endocrine function, and obesity increases the amount of adipose tissue in the body, causing inflammation (Fantuzzi, 2005; Van der Heijden et al., 2015). The weight of adipose tissue was 0.7 (± 0.13) g in the HLBPJ group, 0.6 (± 0.08) g in the HLBCO group, and 0.8 (± 0.04) g in the HLBAG group. Compared to 0.9 (± 0.01) g in the HFD group, body weight was significantly reduced in the fermented liquid administration group, HLBPJ, HLBCO, and HLBAG. In this study, the weight of adipose tissue was significantly increased to 0.9 (± 0.01)g in the HFD group, while it was significantly decreased in the HLBPJ, HLBCO, and HLBAG groups, suggesting that the fermented liquid could be used to prevent obesity by inhibiting lipogenesis and accumulation in the body (Fig. 2C). Colon tissue absorbs water and electrolytes, absorbs fatty acids, stores feces, and excretes feces (McNabney and Henagan, 2017; Yehuda-Shnaidman and Schwartz, 2012). The weight of colon tissue increased to 0.4 (± 0.04)g in the HLBPJ group, 0.5 (± 0.03)g in the HLBCO group, and 0.3 (± 0.02)g in the HLBAG group (Fig. 2D). In this study, the weight of colon tissue decreased to 0.2 (± 0.01)g in the HFD group, but there was no significant difference from the normal group in the fermented liquid administration group. Although the administration of the fermented liquid did not cause weight loss, it was confirmed that it was effective in reducing body fat by suppressing fat accumulation in liver, colon, and adipose tissue.

Measurement of mouse body weight, liver, colon, and adipose tissue in NOR, HFD, HLBPJ, HLBCO, and HLBAG groups. Data are expressed as mean ± the standard error of the mean (n = 6). *p < .05 vs. HFD. NOR:Normal, HFD:High-fat diet, LBPJ:Lactobacillus Paeonia japonica 100mg/kg, LBCO:Lactobacillus Cnidium officinale 100mg/kg, LBAG:Lactobacillus Angelica gigas nakai 100 mg/kg.
Biochemistry analyze
In this study, total cholesterol (TC), triglyceride (TG), HDL and LDL cholesterol, AST, ALT, BUN, and creatinine were diagnosed. Blood total cholesterol levels were 91.0 (± 0.58) mg/dL in the HLBPJ group, 92.2 (± 0) mg/dL in the HLBCO group, and 90.3 (± 0.33) mg/dL in the HLBAG group, and there was no significant difference compared to 96.1 (± 0) mg/dL in the HFD group (Fig. 3A). Blood HDL levels were 87.6 (± 1.38) mg/dL in the HLBPJ group, 90.1 (± 0.65) mg/dL in the HLBCO group, and 87.2 (± 1.62) mg/dL in the HLBAG group, and there was no significant difference compared to 90.7 (± 0.3) mg/d in the HFD group (Fig. 3B). Blood LDL levels were 15.1 (± 0.33) mg/dL in the HLBPJ group, 14.6 (± 0) mg/dL in the HLBCO group, and 16.0 (± 0) mg/dL in the HLBAG group, and there was no significant difference compared to 17.0 (± 0) mg/dL in the HFD group (Fig. 3C). Blood triglycerides were 89.1 (± 0.67) mg/dL in the HLBPJ group, 90.6 (± 0.88) mg/dL in the HLBCO group, and 85.6 (± 0.88) mg/dL in the HLBAG group, and there was no significant difference compared to 94.6 (± 0.5) mg/dL in the HFD group (Müller et al., 2003) (Fig. 3D). Creatinine and BUN were measured to determine their effect on kidney function (Uchino et al., 2012). The blood creatinine concentration was 0.40 (± 0.003) mg/dL in the HLBPJ group, 0.36 (± 0.003) mg/dL in the HLBCO group, and 0.38 (± 0.007) mg/dL in the HLBAG group, showing no significant difference compared to 0.42 (± 0.01) mg/dL in the HFD group (Fig. 3G). Blood creatinine is a waste product produced by the decomposition of creatinine, a compound produced in muscles, and is released into the blood when muscles are damaged, but there is no significant difference in creatinine levels in this study (O'Brien et al., 2016). Blood BUN levels were 18.6 (± 0.006) mg/dL in the HLBPJ group, 17.5 (± 0.012) mg/dL in the HLBCO group, and 17.6 (± 0.003) mg/dL in the HLBAG group, and significantly decreased compared to 30.7 (± 0.009) mg/dL in the HFD group (Fig. 3H). BUN is a protein that metabolizes to ammonia in the body and then metabolizes to urea nitrogen to form urea toxic waste in the liver. In this study, the BUN level was significantly reduced in all fermentation solution administration groups compared to the HFD group (Cindik et al., 2005; She et al., 2023). Blood ALT and AST were measured to check the degree of inflammation or damage to the liver (Marchesini et al., 2008; Scheen and Luyckx, 2002). ALT is an enzyme present in liver cells, and when liver damage occurs, it leaks into the blood, increasing blood levels (Huang et al., 2006; Palipoch and Punsawad, 2013). Blood ALT levels were 33.7 (± 0.33) u/L in the HLBPJ group, 32.5 (± 2) u/L in the HLBCO group, and 35.1 (± 0.67) u/L in the HLBAG group, and there was no significant difference compared to 37.2 (± 0.5) u/L in the HFD group (Fig. 3E). AST is an enzyme that catalyzes the reversible transfer of a-amino groups between aspartic acid and glutamic acid (Nyblom et al., 2004). The blood AST levels were 36.6 (± 3.67) u/L in the HLBPJ group, 35.4 (± 24.5) u/L in the HLBCO group, and 36.7 (± 1.53) u/L in the HLBAG group, and there was no significant difference compared to the HFD group (Fig. 3F).
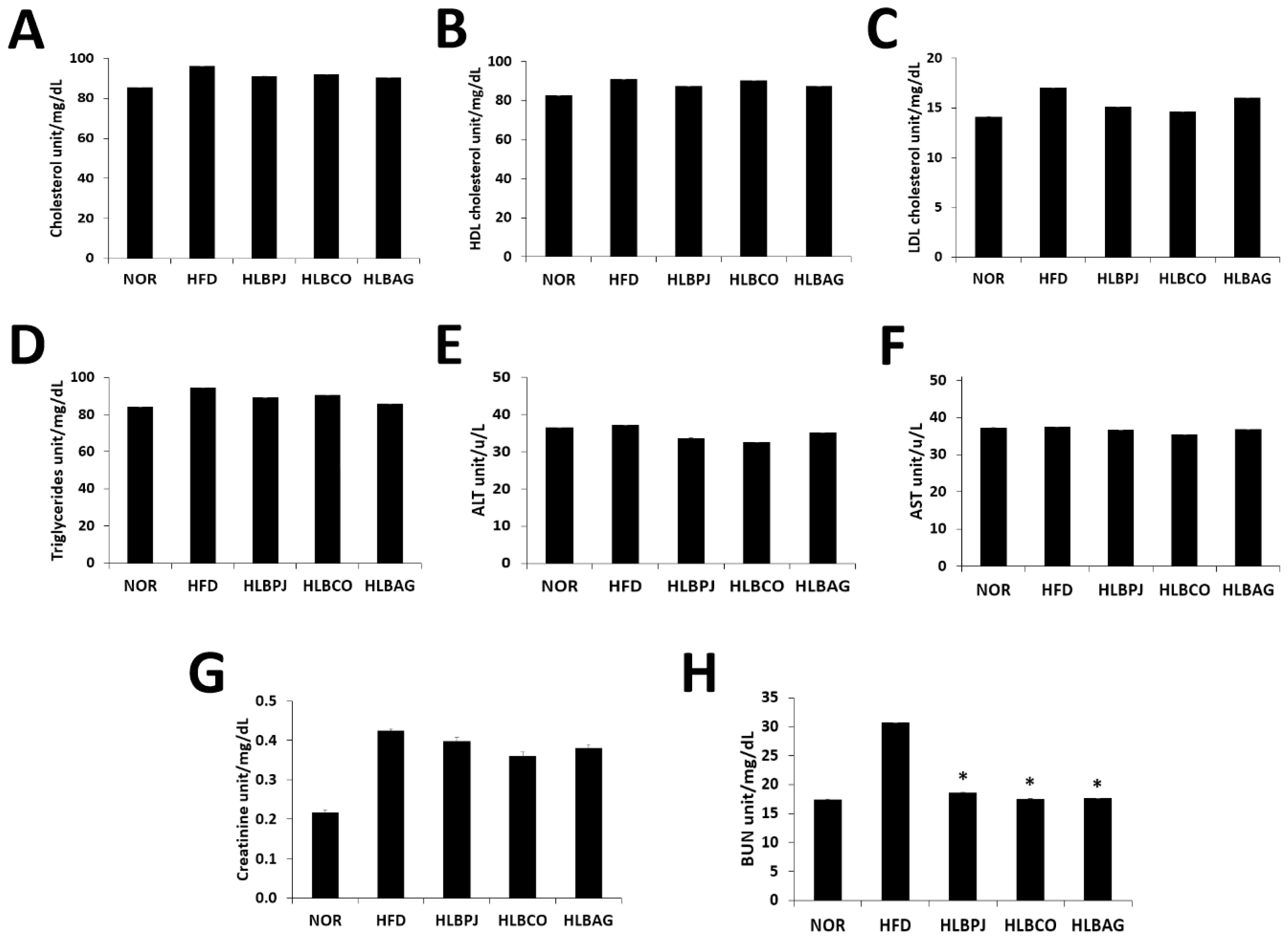
Effect of NOR, HFD, HLBPJ, HLBCO, and HLBAG on activity of serum lipid levels focus high-fat diet induced mice. Data are expressed as mean±the standard error of the mean (n = 6). *p < .05 vs. HFD. NOR:Normal, HFD:High-fat diet, LBPJ:Lactobacillus Paeonia japonica 100 mg/kg, LBCO:Lactobacillus Cnidium officinale 100 mg/kg, LBAG:Lactobacillus Angelica gigas nakai 100 mg/kg.
Enzyme linked immunosorbent assay (ELISA)
To confirm the effect of reducing inflammatory cytokines using mice liver, IL-6 and TNF-α production were measured using enzyme-linked immunosorbent assay. The IL-6 measurements showed that the HLBPJ group produced 1.64 (± 0.01) pg/mL, the HLBCO group 1.82 (± 0.008) pg/mL, and the HLBAG group produced 1.77 (± 0.032) pg/mL less cytokine than the HFD group 2.16 (± 0.019) pg/mL (Fig. 4A). As a result of TNF-α measurements, the HLBPJ group was 1.47 (± 0.012) pg/mL, the HLBCO group was 1.58 (± 0.024) pg/mL, the HLBAG group was 1.6 (± 0.021) pg/mL, and cytokine production was reduced compared to 2.43 (± 0.008) pg/mL of the HFD group (Fig. 4B). The production of the inflammatory cytokines TNF-α and IL-6 in the mice liver increased significantly in the HFD group due to high fat diets and tended to decrease in the fermented liquid administration group. IL-6 did not differ significantly between NOR and HFD groups and fermented liquid administration groups, but TNF-α decreased in the fermented liquid administration group excluding NOR and HFD groups, and significantly decreased in the HLBAG group. In this study, the fermented liquid has an anti-inflammatory effect that suppresses inflammation of the liver in the body.
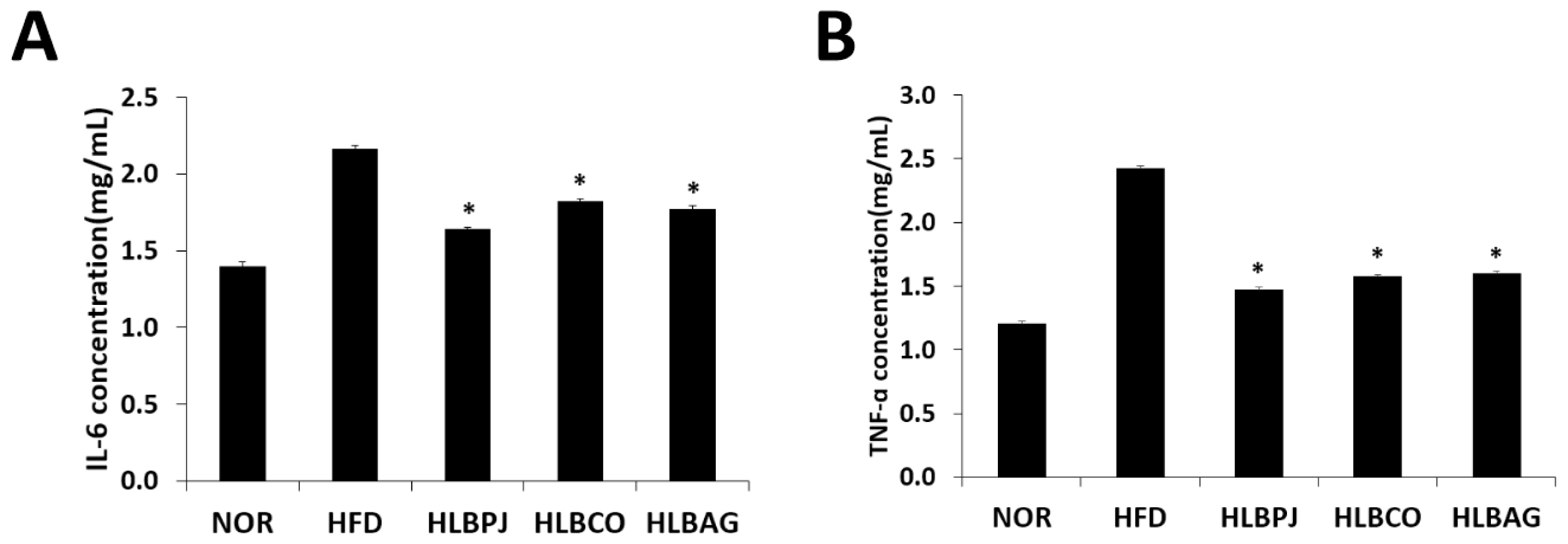
Effects of (A)IL-6 and (B)TNF-α production in the liver induced by high-fat diet. Data are expressed as mean±the standard error of the mean (n = 6). *p < .05 vs. HFD. NOR:Normal, HFD:High-fat diet, LBPJ:Lactobacillus Paeonia japonica 100 mg/kg, LBCO:Lactobacillus Cnidium officinale 100 mg/kg, LBAG:Lactobacillus Angelica gigas nakai 100 mg/kg.
Reverse transcription-polymerase chain reaction (RT-PCR)
Using the liver of mice, changes in the expression of fat-related cytokine mRNA genes and antioxidant and inflammatory cytokine mRNA genes were checked. The primers listed in (Table 3) were used in the experiments. Genes related to fat synthesis: PPARα (peroxisome polymer-activated receptor α), PPARγ (peroxisome polymer-activated receptor γ), CPT-2 (carnitine palmitoyl transferase 2), HSL (hormone-sensitive lipase), FABP4 (fatty Acid-Binding Protein 4) and ACC (acetyl-CoA carboxylase) was analyzed. As a result of PPARα analysis, compared to 1.49 ng/g in the HFD group, it decreased to 1.16 ng/g in the HLBPJ group, 0.61 ng/g in the HLBCO group, and 0.3 ng/g in the HLBAG group, and it decreased significantly in the HLBCO group and the HLBAG group (Fig. 5C). As a result of PPARγ analysis, the HLBPJ group was 1.23 ng/g, the HLBCO group was 0.68 ng/g, and the HLBAG group was 0.34 ng/g, which was significantly reduced in the HLBCO and HLBAG groups compared to 1.73 ng/g in the HFD group (Fig. 5B). Lipid metabolites are regulated by PPAR, and PPARα is expressed within hepatocytes, regulating fatty acid oxidation and lipid metabolism, reducing liver fat, and improving inflammation (Bougarne et al., 2018; Kersten, 2014). PPAR γ is a transcription factor that regulates adipocyte differentiation and lipid accumulation in adipose and muscle tissues (Inoue et al., 2005; Jones et al., 2005). In this study, mRNA expression of PPARα and PPARγ in the fermented liquid administration group can be significantly downregulated in the HLBAG group, thereby inhibiting adipocyte differentiation and lipid accumulation. As a result of the CPT-2 analysis, 1.02 ng/g in the HLBPJ group, 0.42 ng/g in the HLBCO group, and 0.42 ng/g in the HLBAG group decreased compared to 1.81 ng/g in the HFD group, and significantly decreased in the HLBCO and HLBAG groups (Fig. 5D). CPT-2 is an enzyme that regulates fatty acid oxidation in mitochondria, catalyzing dissociation of acyl-carnitine and recombination with acyl-CoA, which is involved in mitochondrial substrate fatty acid metabolism (Jiang et al., 2023; Thuillier et al., 2023). HSL is a hormone-sensitive lipolytic enzyme and is the most important enzyme in the neutrophil degradation phase of adipose tissue, and when HSL is activated, fat accumulation decreases (Althaher, 2022; Lan et al., 2019). As a result of HSL analysis, the HLBPJ group was 1.44 ng/g, the HLBCO group was 0.94 ng/g, and the HLBAG group was 0.17 ng, down from 1.76 ng/g in the HFD group, and down significantly in the HLBAG group (Fig. 5E). In this study, it was confirmed that mRNA of HSL was under-regulated in the fermented liquid administration group, and in particular, the HLBAG group was significantly reduced to increase fatty acid oxidation in adipose tissue and to degrade triglycerides to inhibit body fat accumulation. FABP4 is expressed in adipocytes, where fatty acids are delivered to adipocytes, and fatty acids are accumulated in the form of triglycerides (Furuhashi, 2019; Liu et al., 2022). As a result of analysis with FABP4, it was significantly decreased in HLBPJ group 0.39 ng/g, HLBCO group 0.54 ng/g, and HLBAG group 0.28 ng/g compared to HFD group 1.68 ng/g (Fig. 5F). In this study, mRNA expression was significantly reduced in the fermented liquid administration group, thereby inhibiting the differentiation and accumulation of neutral lipids. ACC regulates fatty acid metabolism, produces malonyl-CoA products when ACC is activated to become a new fatty acid component, and inhibits β-oxidation of carnitine by inhibiting the movement of fatty acyl groups from acyl-CoA to carnitine by carnitine acyltransferase (Kashyap et a l., 2023; Shi and Tu, 2015). As a result of ACC analysis, compared to 2.14 ng/g in the HFD group, 1.03 ng/g in the HLBPJ group, 0.82 ng/g in the HLBCO group, and 0.43 ng in the HLBAG group were significantly reduced (Fig. 5A). In this study, ACC mRNA expression was downregulated in the fermented liquid administration group, so obesity could prevent resistance. IL-1 is a cytokine produced by activated mononuclear cells that mediate inflammatory responses, and IL-1β can stimulate inflammatory cells by activating small amounts of CD4 T cells and B cells, but if IL-1 is made excessively, it acts as a hormone and causes fever and acute reactions (González et al., 2022; Mantovani et al., 2019; Teufel et al., 2022). In this study, the expression of IL-1β mRNA was lowered in the fermented liquid administration group, and in particular, the HLBAG group reduced the inflammatory response by inhibiting cytokine involved in the inflammatory response (Fig. 5H). Antioxidant-related genes Mn SOD and Cu/Zn SOD are analyzed for expression (Pérez-Torres et al., 2021). Mn SOD is a mitochondrial protein that converts hydrogen peroxide radicals formed by cellular respiration into hydrogen peroxide (Sato et al., 2022). Mn SOD is a mitochondrial protein that converts hydrogen peroxide radicals formed by cellular respiration into hydrogen peroxide (Liu et al., 2013). Cu/Zn SOD is an antioxidant enzyme that protects cells from oxidative stress (Lewandowski et al., 2019; Jomova et al., 2022). In this study, it was confirmed that Mn SOD and Cu/Zn SOD mRNA expression were downregulated in the fermented liquid administration group, especially in the HLBAG group (Figs. 5G, 5I).
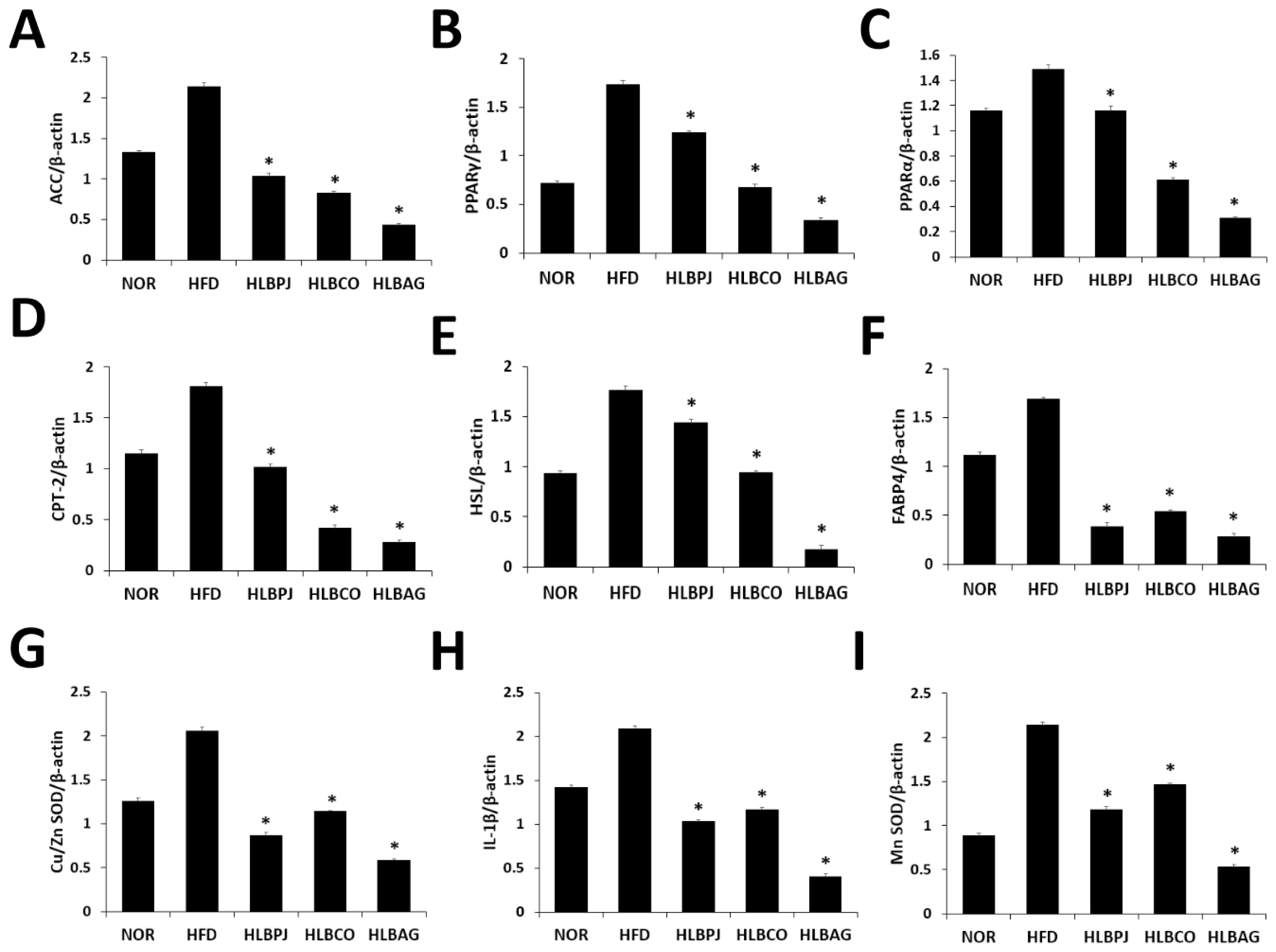
Inhibitory effects of NOR, HFD, HLBPJ, HLBCO, and HLBAG on mRNA expression of inflammatory cytokines. Expression of genes associated adipocyte differentiation and accumulation in the liver. Data are expressed as mean±the standard error of the mean (n = 6). *p < .05 vs. HFD. NOR:Normal, HFD:High-fat diet, LBPJ:Lactobacillus Paeonia japonica 100 mg/kg, LBCO:Lactobacillus Cnidium officinale 100 mg/kg, LBAG:Lactobacillus Angelica gigas nakai 100 mg/kg.
Conclusion
In this study, we confirmed that LBPJ, LBCO, and LBAG, fermentants obtained by fermenting PJ, CO, and AG extracts using Lactobacillus, decomposes or modifies drug components through the metabolism of Lactobacillus and produces beneficial compounds of Lactobacillus itself. When the fermented liquid was administered, the expression of genes involved in liver fat synthesis was significantly reduced, and the liver fat content in the body of mice fed a high-fat diet was significantly reduced. The reduction of inflammatory cytokines in fermentants LBPJ, LBCO, and LBAG helps regulate lipid synthesis and expression of inflammation-related genes, thereby increasing hepatic β-oxidation of fatty acids. Fermented liquid has the effect of inhibiting the growth of fat cells, and in particular, LBCO fermented liquid helps prevent obesity by reducing body fat and blood sugar levels.