Germination Characteristics and Seedling Growth of Pseudolysimachion nakaianum (Ohwi) T. Yamaz. Seeds According to Light Quality Type
Article information
Abstract
Background and objective
This study was conducted to understand the effect of different quality of light-emitting diodes (LED) on the germination characteristics and growth of Pseudolysimachion nakaianum.
Methods
The LED light irradiation was divided into white, red, blue, and red:blue (1:1). To investigate the germination characteristics, 30 seeds were placed in a Petri dish in three replicates for 8 days. Average germination rate, germination speed, germination uniformity, and average germination time were measured. To investigate the growth of seedlings, plant height, plant width, leaf length, leaf width, leaf number, fresh weight, dry weight and T/R ratio were examined 6 weeks after sowing, and chlorophyll fluorescence (maximum quantum yield, electron transport rate) was measured.
Results
The final germination rate and germination speed were highest at red:blue (1:1) LED irradiation. Regarding growth and chlorophyll fluorescence measurements, red:blue LED showed the best growth results, with no significant difference in Fv/Fm between different types of light quality, and the electron transport rate was the highest under red:blue LED irradiation. Also, total fresh/dry weight and T/R ratio were highest for red:blue LED, it is presumed to promote the biomass of entire plant.
Conclusion
The results proved that Pseudolysimachion nakaianum showed red LED conditions promoted germination, but cultivation under red:blue LED conditions is judged to be suitable for seedling growth and photosynthesis.
Introduction
Native plants grow in their natural habitats without any human intervention (Song et al., 2015). There are 3,940 known species of native plants distributed in South Korea (KPNI, 2022a). Native plants high capacity for environmental adaptation and disease tolerance (Ryu, 2004) and their with ornamental values are used as cut flowers and for garden landscaping. However, compared to imported plants, information on the cultivation and management of native plants is insufficient, so there is a need to continuously conduct various studies on native plants. The genus Pseudolysimachion, in the Scrophulariaceae family, is presented with 16 species, including Pseudolysimachion (=Veronica) pusanensis, P. kiusianum, and P. nakaianum, in the flora of South Korea (KPNI, 2022b). P. nakaianum, an endemic species to South Korea, grows naturally only on Ulleungdo Island and is designated as an endangered species by the International Union for Conservation of Nature (IUCN) (KPNI, 2022c). P. nakaianum are approximately 30 cm in height, and its ovoid leaves are 3.5–5 cm in length with sharply toothed edges and alternate arrangement. In addition, compared to other naturally growing plants of the Pseudolysimachion genus that flower during July–September, P. nakaianum develops racemose inflorescence one or two months earlier, from June to July. The showy light-blue flower petals are of great ornamental value. To date, studies on P. nakaianum have investigated the changes in growth characteristics under artificial shading and the seed dormancy type for propagation (Song et al., 2019; Kwon et al., 2020). However, to the best of our knowledge, no study is available regarding the method of assessing seedling growth characteristics of P. nakaianum. Therefore, a need to develop a method for assessing seedling growth, which will help in the conservation of this rare and endemic plant and its utilization in landscaping.
Plug seedling production is a method that uses a plug tray, which not only facilitates the growth of plants but also controls the production time through systematized operation from irrigation to fertilizer application and environmental management of seedlings (RDA, 2023). The advantages of the plug tray method of seedling cultivation are reduced loss of seeds, production of uniform seedlings, and mass production(Ito, 1992). Therefore, this method lowers labor via efficient planning and specialization of production.
Light is one of the environmental factors influencing plant growth by engaging in morphogenesis, and plants respond differently to varying light intensity and quality (Zheng and Van Labeke, 2017). Notably, the wavelengths covering red light (600–700 nm) and blue light (400–500 nm) are absorbed by chlorophylls a and b and have a critical effect on plant growth (Terashima et al., 2009). They also serve as an essential energy source through various reactions in photoreceptors, such as phytochromes and cryptochromes, thereby inducing plant growth and development (Barros et al., 2011; Lin et al., 2013; Son et al., 2016).
Light-emitting diodes (LEDs) are widely used as an artificial light source in environmentally controlled systems such as plant factories (Lin et al., 2013). They are convenient to use, readily form a spectrum of wavelengths, have a long lifespan, generate little heat, and are highly durable. In addition, LEDs can be combined to produce wavelengths that meet the light requirement of plants and affect plant morphological and metabolic properties, which possibly render high-quality crop production (Massa et al., 2008; Morrow, 2008). While studies have used LEDs to define the suitable wavelengths for plant growth, most of the previous studies have focused on edible vegetables or fruits (Cho et al., 2008; Lee et al., 2010; Cha et al., 2013; Kim and You, 2013). Nevertheless, with the recent increase in the demand and interest in wild flowers, studies are investigating LED-based seedling cultivation (Oh et al., 2019; Lee et al., 2020; Oh, 2021). The type of light quality determines plant growth and seed germination characteristics. It is, therefore, of particular importance to identify light quality and the respective wavelengths that are suitable for each plant species, to ensure uniformity and rapid growth of plants. Thus, this study aimed to investigate P. nakaianum, an endemic and endangered plant species in South Korea, in a phytotron system with a controlled environment for temperature and humidity, to identify the effects of LED light quality on seed germination characteristics and plant growth, electron transport rate (ETR), and maximum quantum yield as the chlorophyll fluorescence mechanism, and to provide basic information.
Research Methods
Test material
P. nakaianum seeds were collected from the greenhouse in the Useful Plant Cultivation Center located in Yongmunmyeon, Yangpyeong-gun, Gyeonggi-do, South Korea (37°28′ 45.2″N, 127°35′51.4″E) in November 2018. The seeds were stored at 4 °C in a dry place for 27 months after harvest.
Seed germination characteristics under different light quality
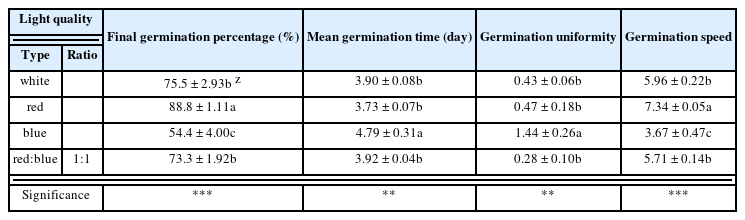
P. nakaianum seeds were disinfected in 1,000 mg · L−1 Benomyl (FarmHannong, Seoul, Korea) for 24 h. Next, the seeds were placed in a Petri dish lined with two layers of filter paper (Whatman No.1, GE Healthcare, Buckinghamshire, UK), and distilled water was added. Each Petri dish contained 30 seeds; three replicates of 30 seeds each were prepared for each treatment. The experimental conditions were maintained at 25 ± 1 °C, 70 ± 5% humidity, and 9/15 h (day/night) photoperiod in a sealed plant growth system. The seeds were cultured for 8 days on a shelf, under controlled red LED (660 nm), blue LED (450 nm), red:blue (1:1) LED (450, 660 nm), or white LED (406 – 770 nm, 458 nm at maximum) (Fig. 1). Petri dish and plug tray between LED bar spacing was 40 cm. And light intensity of light quality was 100 ± 10 μ mol · m−2 · s−1, respectively. The germination rate was monitored each day. Germination was defined as ≥ 1 mm root growth, and the final germination rate (FGR), mean germination time (MGT) (Edwards, 1934), germination uniformity (GU), and germination speed (GS) were estimated using the following equations:

Relative spectral distribution of the light emitting diodes used in this study. A, Red; B, Blue; C, Red + Blue; D, White led.
(N: total number of germinated seeds, S: total number of seeds, Nx: number of germinated seeds on the day of investigation, Tx: number of days after explanting)
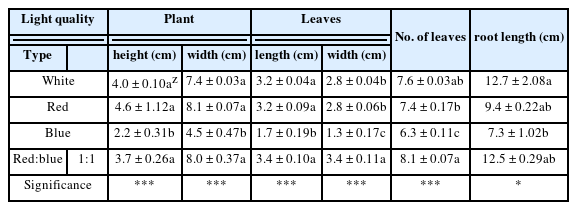
Seedling growth under different light quality
To examine the effects of light quality on the growth of P. nakaianum, a plug tray of 128 cells (21 mL/cell; Bumnong Co., Ltd, Korea) of W× L × H: 280 × 540 × 45 mm was used. Each cell was filled with topsoil (Baroker, Seoulbio, Korea), and 2 – 3 seeds were sown per cell. Each treatment was performed in 3 repetitions of 15 plants A sub-irrigation system was used for watering twice a week to prevent the topsoil from drying. Thinning was performed 10 days after sowing to ensure uniform plant growth in the tray. The experiment was conducted for 6 weeks at the same location as the seed germination experiment.
Chlorophyll fluorescence under different light quality
To analyze the chlorophyll fluorescence, six leaves were selected per treatment after 4 weeks. The leaves were placed in the dark for 30 min adaptation, and using a chlorophyll fluorescence analyzer (Maxi Imaging-PAM-series, Heinz Walz GmbH, Effeltrich, Germany), the maximum quantum yield of PSII (Fv/Fm) and the ETR were measured as indicators of chlorophyll fluorescence.
Investigation of plant growth and statistical analysis
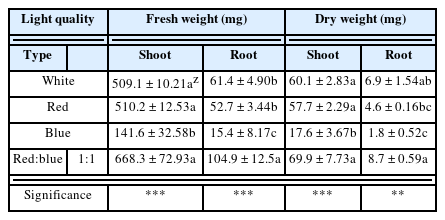
To determine the growth of P. nakaianum, the plant height, plant width, leaf length, leaf width, leaf number, root length, and fresh, dry weights, and T/R ratio of the above- and below-ground parts of the plant were measured 6 weeks after irradiation. The plant height was measured as the length from the soil surface to the topmost part of the plant, and the plant width was measured as the length between the ends of the largest leaf. The length and width of the largest leaf were measured, and for the leaf number, rooted leaves were counted. The root length was measured as the longest one. The fresh weight of the above- and below-ground parts were measured immediately after the experiment using an electronic scale (Shimadzu Analytical Balance AUW2200, Shimadzu, Japan). The dry weight was measured after 72 h of drying at 70 °C in a constant-temperature dryer.
For statistical analysis, the SPSS 20 (IBM Corporation, Armonk, NY, USA) was used to perform analysis of variance (ANOVA) with Tukey’s honestly significant difference (HSD) pos-hoc test (p = .05) in the cases showing statistically significant differences. The graphs were drawn using SigmaPlot 12.5 (Systat, Palo Alto, CA, USA).
Results and Discussion
Seed germination characteristics under different light quality
The germination characteristics of P. nakaianum were analyzed according to the LED light quality. In all treatment groups, germination commenced on day 3 and ceased after day 7. The FGR under red LED, white LED, red:blue (1:1) LED, and blue LED was 88.8, 75.5, 73.3, and 54.4%, respectively (Table 1). The GS was also the highest under the red LED conditions at 7.34, followed by white LED, red:blue (1:1) LED and blue LED at 5.96, 5.71, and 3.67, respectively. The MGT indicated that germination was the slowest under blue LED conditions at 4.79 days, but it was the fastest under red LED conditions at 3.73 days, followed by white LED (3.90 days) and red:blue (1:1) LED (3.92 days); no significant difference was found across the three conditions. GU was the highest under blue LED conditions at 1.44, which indicated a lack of uniformity; uniformity was mostly observed under red LED, white LED, and red:blue (1:1) LED conditions at 0.47, 0.43, and 0.28, respectively. Numerous studies have examined the effect of light quality on seed germination. In Cynanchum wilfordii seeds, the GR and GS were higher with red LED treatment than with blue LED treatment (Yoo et al., 2013). The initial GR and GS of the seeds of Lactuca sativa and Brassica napus were lower under red LED than under blue LED (Cho et al., 2008; Hwang et al., 2008). In addition, Lee et al. (2020) reported that the group of Veronica rotunda seedlings irradiated with red LED demonstrated outstanding initial growth, but the group irradiated with blue LED showed growth inhibition, which was attributed to the variation in initial GR at different light qualities. As each plant species exhibits a unique level of sensitivity to light quality, seed germination characteristics vary (Choi et al., 2019). In P. nakaianum, irradiation with red LED resulted in overall outstanding germination characteristics based on the mean values of GS, and MGT, although the three treatment conditions, i.e., red LED, white LED, and red:blue (1:1) LED, did not demonstrate statistically significant different. However, FGR and GS were significantly difference. This is presumed to be due to the inhibitory effects of blue LED on seed growth and the high sensitivity of P. nakaianum seeds to red LED, which resulted in facilitated germination.
Seedling growth under different light quality
The growth of P. nakaianum under different light quality was examined for 6 weeks. The plant height, plant width, leaf length, leaf width, and leaf numbers were affected by the light quality at a significance level of 0.001. The plant height and plant width were the highest at 4.6 cm and 8.1 cm, respectively, under red LED; 4.0 cm and 7.4 cm, respectively, under white LED; and 3.7 cm and 8.0 cm, respectively, under red:blue (1:1) LED, with no significant variation across the three conditions. The lowest values of plant height and width at 2.2 cm and 4.5 cm, respectively, were found under blue LED (Table 2). Under red:blue LED, the leaf length and leaf width were identical at 3.4 cm. Under white and red LED, the leaf length and leaf width were 3.2 cm and 2.8 cm, respectively. Under blue LED, the leaf length and leaf width were 1.7 cmand 1.3 cm, respectively, indicating the smallest leaf size. The leaf number was 8.1 under red:blue (1:1) LED, indicating the largest number of rooted leaves, followed by that under white LED at 7.6, red LED at 7.4 and blue LED at 6.3. The fresh weight and dry weight varied significantly (p = .01 and p = .001) with the highest levels observed under red:blue (1:1) LED (Table 3). The mean fresh weight of the above-ground parts was the highest at 668.3 mg under red:blue (1:1) LED and the lowest under blue LED, and no significant difference was found between red LED and white LED. The fresh weight of the below-ground parts was also the highest at 104.9 mg under red:blue (1:1) LED, followed by red LED, white LED, and blue LED. The dry weight of the above-ground plant parts was the highest under red:blue (1:1) LED, followed by that under white LED, red LED, and blue LED, although no significant difference was noted between red LED and white LED. The dry weight of the below-ground plant parts was also the highest under red:blue (1:1) LED, followed by that under white LED, red LED, and blue LED, but with a significant difference between white LED and red LED. However, total weight of fresh and dry plant were the highest at red:blue (1:1) LED and each of fresh and dry T/R ratio was found to lowest than other light quality (Fig. 2). Also, each of fresh and dry total weight and T/R ratio had significant difference. In general, red LED has been shown to induce over-growth by increasing the plant height, whereas blue LED prevents an increase in plant height (McMahon et al., 1991; Rajapakse and Kelly, 1992). This is inagreement with the findings of previous studies reporting the positive effect of single irradiation of red LED on initial growth and plant height (Yoo et al., 2013; Kim et al., 2018; Lee et al., 2020). In addition, blue LED is known to activate the photoreceptor cryptochrome 1, and cryptochromes inhibit plant growth by regulating hypocotyl elongation (Lin et al., 1996; Folta and Spalding, 2001). Hence, the inhibited growth of P. nakaianum with blue LED is presumed to be due to the effect of cryptochrome 1. In addition, research results have shown that treatment with red:blue (1:1) LED increases the leaf width and the biomass of the entire plant, enabling the production of high-quality plants when compared to treatments with only red or blue LED (Lee et al., 2010; Kim and You, (Lee et al., 2010; Kim and You, 2013; Kim and You, 2013; Lee et al., 2016). In the case of P. nakaianum, likewise, the synergistic effects of red and blue LED appear to promote the biomass of the entire plant and it is presumed that red:blue LED impact to plant development. Thus, the production of high-quality P. nakaianum seedlings is predicted with the combination of the red and blue LED. In line with previous studies investigating plant growth according to the varying ratio of red and blue LED (Cha et al., 2013; Seong et al., 2015; Lee et al., 2016), further studies on P. nakaianum are needed to investigate the differences in growth under varying ratios of red and blue LED conditions.
Effects of different light quality types on the growth of Pseudolysimachion nakaianum at 42 d after treatment
Effects of different light quality on fresh weight and dry weight of Pseudolysimachion nakaianum at 42 d after treatment
Chlorophyll fluorescence under different light quality
Chlorophyll fluorescence is widely used as a non-invasive technique to measure the details of photosynthetic reactions under environmental stress and genetic mutation (Murchie and Lawson, 2013). The maximum quantum yield (Fv/Fm), in particular, is an indicator of the potential of dark-adapted leaves to perform photosynthesis, and it is thus used as a marker of the level of damage in the PSII complex due to photoinhibition or photodamage (Brestic and Zivcak, 2013). The maximum quantum yield of P. nakaianum was 0.86, 0.84, 0.85, and 0.86 under white LED, red LED, blue LED, and red:blue (1:1) LED, respectively, and their have no significant differences (Fig. 3A), presumably because light quality type does not affect chlorophyll despite its effect on plant growth. The ETR indicates the rate of electron transport across the leaves to reflect the actual speed of electron flow and is thus viewed as a photosynthetic parameter regarding the efficiency of photosynthesis (Llorens et al., 2003; Ambede et al., 2012). The ETR of P. nakaianum was the highest at 5.65 under the red:blue (1:1) LED, followed by red LED (5.38), white LED (4.36), and blue LED (2.86) (Fig. 3B). This is presumed to be due to the effect of light quality on the electron activities inside the chlorophyll. Thus, despite the lack of direct damage to chlorophyll, plant growth is ultimately affected.

Effects of different light quality types on the Fv/Fm (A) and electron transport rate (ETR) (B) of Pseudolysimachion nakaianum, 42 d after treatment. Vertical bars represent SE (n = 6). Mean values with different letters are significantly different according to Tukey’s honestly significant difference (HSD) test at p = .05.
The results of this study collectively suggest that the seed germination characteristics and plant growth vary according to the type of light quality. Seed germination characteristics was high to the red LED conditions than other. In contrast, blue LED treatment led to overall low levels of germination thus exhibiting an inhibitory effect. Also, plant height and width were the highest in red LED, but length, width, and number of leaf, and fresh weight and dry weight were the highest besides the T/R ratio of fresh weight and dry weight came out statistically significant in red:blue LED. And the lack of significant variation in Fv/Fm indicated that the chlorophyll was intact across all light quality types. However, the ETR as the indicator of electron transport rate across the leaves varied according to the type of light quality. It was the highest under red:blue LED, suggesting that the ability of the leaf to transport electrons affects plant growth. Furthermore, just as studies are being conducted on the growth of P. nakaianum depending on the mixing ratio of red and blue LEDs (Cha et al., 2013; Seong et al., 2015; Lee et al., 2016), it seems necessary to conduct research by mixing various ratios of red and blue LEDs in the future.
Conclusion
This study was conducted to understand the effect of different quality of light-emitting diodes (LED) on the germination characteristics and growth of Pseudolysimachion nakaianum. The LED light irradiation was divided into white, red, blue, and red:blue (1:1). To investigate the germination characteristics, 30 seeds were placed in a Petri dish in three replicates for 8 days. Average germination rate, germination speed, germination uniformity, and average germination time were measured. To investigate the growth of seedlings, plant height, plant width, leaf length, leaf width, leaf number, fresh weight, dry weight and T/R ratio were examined 6 weeks after sowing, and chlorophyll fluorescence (maximum quantum yield, electron transport rate) was measured. The final germination rate and germination speed were highest at red:blue (1:1) LED irradiation. Regarding growth and chlorophyll fluorescence measurements, red:blue LED showed the best growth results, with no significant difference in Fv/Fm between different types of light quality, and the electron transport rate was the highest under red:blue LED irradiation. Also, total fresh/dry weight and T/R ratio were highest for red:blue LED, it is presumed to promote the biomass of entire plant. The results proved that Pseudolysimachion nakaianum showed red LED conditions promoted germination, but cultivation under red:blue LED conditions is judged to be suitable for seedling growth and photosynthesis.