Shoot Growth and Virus Elimination by Antiviral Agents in Node Cultures of Three Rose Cultivars
Article information
Abstract
Background and objective
This study was carried out to examine the effects of antiviral agents on shoot growth and virus elimination during node culture in rose 'Deep Purple', 'Natal Briar' and 'Pink Beauty' infected with TRSV and ArMV.
Methods
Three varieties of roses confirmed to be infected with TRSV and ArMV through ImmunoStrip® Tests were node-cultured, and the medium was treated with antiviral agents ribavirin and vidarabine at 0, 10, 20, and 40 mg · L−1. After 60 days of culture, shoot growth and virus infection rates were evaluated.
Results
In 'Deep Purple', vidarabine treatment inhibited shoot growth and survival rate more than ribavirin, and had no effect on virus elimination. Treatment of 20 mg · L−1 ribavirin was no significant difference in shoot growth and survival rate compared to control, and TRSV and ArMV infection rates were lowered to 25% and 0%, respectively. In 'Natal Briar', treatment of 10–20 mg · L−1 ribavirin showed no statistical difference in shoot growth compared to the control, but the infection rates of TRSV and ArMV were 100%. The shoot growth and survival rate were greatly suppressed in treatment of 10 mg · L−1 vidarabine, but both TRSV and ArMV infection rates were 50%. In 'Pink Beauty', the higher the treatment concentration of ribavirin and vidarabine, the more the shoot growth was suppressed. Ribavirin had no effect on TRSV elimination, and 20 mg · L−1 vidarabine treatment showed that both TRSV and ArMV infection rates were 50%.
Conclusion
Depending on the type and concentration of the antiviral agent, the elimination effects of viruses were different for each variety of rose. It was most effective to treat 20 mg · L−1 ribavirin in 'Deep Purple', 10 mg · L−1 vidarabine in 'Natal Briar', and 20 mg · L−1 vidarabine in 'Pink Beauty' in media for TRSV and ArMV elimination.
Introduction
Roses, with their variegated colors and beautiful flower shape, are the most favored cut flowers by consumers around the world, and in Korea as well, are the most sold flower with up to 53.6 billion won in production cost in 2021 (MAFRA, 2022). Diseases that most commonly occur in roses include powdery mildew, gray mold, downy mildew, crown gall caused by bacteria, and others caused by viruses. Viruses found in roses include apple mosaic virus (ApMV), arabis mosaic virus (ArMV), impatiens necrotic spot virus (INSV), prunus necrotic ringspot virus (PNRSV), tobacco streak virus (TSV), tobacco ringspot virus (TRSV), tomato ringspot virus (ToRSV), and tomato spotted wilt virus (TSWV) (Ghotbi et al., 2005; Horst and Cloyd, 2007). When a rose is infected with a virus, symptoms such as yellow-stained or twisted leaves, circular yellow spots, and yellowish-white stripes appear on the plant, accompanied by a slowed growth rate and a decrease in the number of flowers (McDaniel et al., 1971; Goldberg, 2006). Plants that are reproduced through vegetative propagation may become infected by viruses during the cutting or grafting process. And since there is no way to control these infections, methods for producing virus-free plants have been developed.
To produce virus-free plants, methods such as heat treatment using temperature around 37°C, cold treatment using temperature around 4°C, chemical treatment using antiviral agents, and meristem culture are utilized. Antiviral agents include ribavirin (1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide), vidarabine (9-β-D-arabinofuranosyladenine), DHT (2,4-Dioxohexahydro-1,3,5-triazine), and have been reported to be effective in eliminating viruses in Vitis vinifera (Kim et al., 2003; Hu et al., 2021), Pyrus pyrifolia (Cho et al., 2016), Prunus persicae (El-Dougdoug et al., 2010), Solanum tuberosum (Yi et al., 2003; Dhital et al., 2008), Cnidium officinale (Kim and An, 2022), Manihot esculenta (Kidulile et al., 2018), Lilium (Seo et al., 1999), and Freesia hybrida (Choi et al., 2016).
Roses are usually propagated through cutting or grafting. 'Natal Briar' is the most widely used variety as a rootstock worldwide, and is also used as a rootstock for 'Deep Purple' and 'Pink Beauty' bred in Korea (Kim et al., 2020; Kwon, 2023). However, it has been reported that these varieties have been infected with viruses such as TRSV and ArMV (Roh and Yoo, 2023). Therefore, it is necessary to develop methods for producing virus-free plants for these varieties. This study was carried out to examine the effects of antiviral agents on shoot growth and virus elimination during node culture in rose 'Deep Purple', 'Natal Briar' and 'Pink Beauty' infected with TRSV and ArMV.
Research Methods
In this study, among rose 'Deep Purple', 'Natal Briar', and 'Pink Beauty' cultivated in pots in a greenhouse, plants diagnosed as being infected with TRSV and ArMV through ImmunoStrip® Tests (Agdia, Inc., Indiana, USA) were used as experiment materials.
Stems of the three rose varieties were collected., and then the leaves were removed. The collected stems were surface-sterilized with a 2% NaOCl solution for 15 minutes, and rinsed with sterile water for three times in clean bench. And then nodes containing one lateral bud were cut into 6~7 mm length size and cultured with three replicates of 5 each in petridish supplemented with medium. MS basal medium supplemented with 3% sucrose and 0.8% agar was used for node culture, and pH adjusted to 5.8. Antiviral agents, ribavirin and vidarabine (Sigma-Aldrich, Co., USA) filtered through Nalgene® syringer filter (pore size 0.2μm, diameter 13mm) were treated in MS media at concentrations of 0, 10, 20, 40 mg · L−1, respectively. Cultures were maintained at 25°C air temperature in a culture room with a16-h photoperiod under 30 μmol · m−2 · s−1 photosynthetic photon flux density provided by fluorescent lamp. After 60 days of treatment, the shoot length, number of shoots and leaves, and survival rate of roses were examined, along with the infection rate of TRSV and ArMV through ImmunoStrip® Tests.
Data collected on shoot growth, survival rate, and infection rate were examined through ANOVA (analysis of variance) using IBM SPSS Statistics (Version 23.0 software, IBM Corp., USA). Means were separated by Duncan's multiple range test at p < .05.
Results
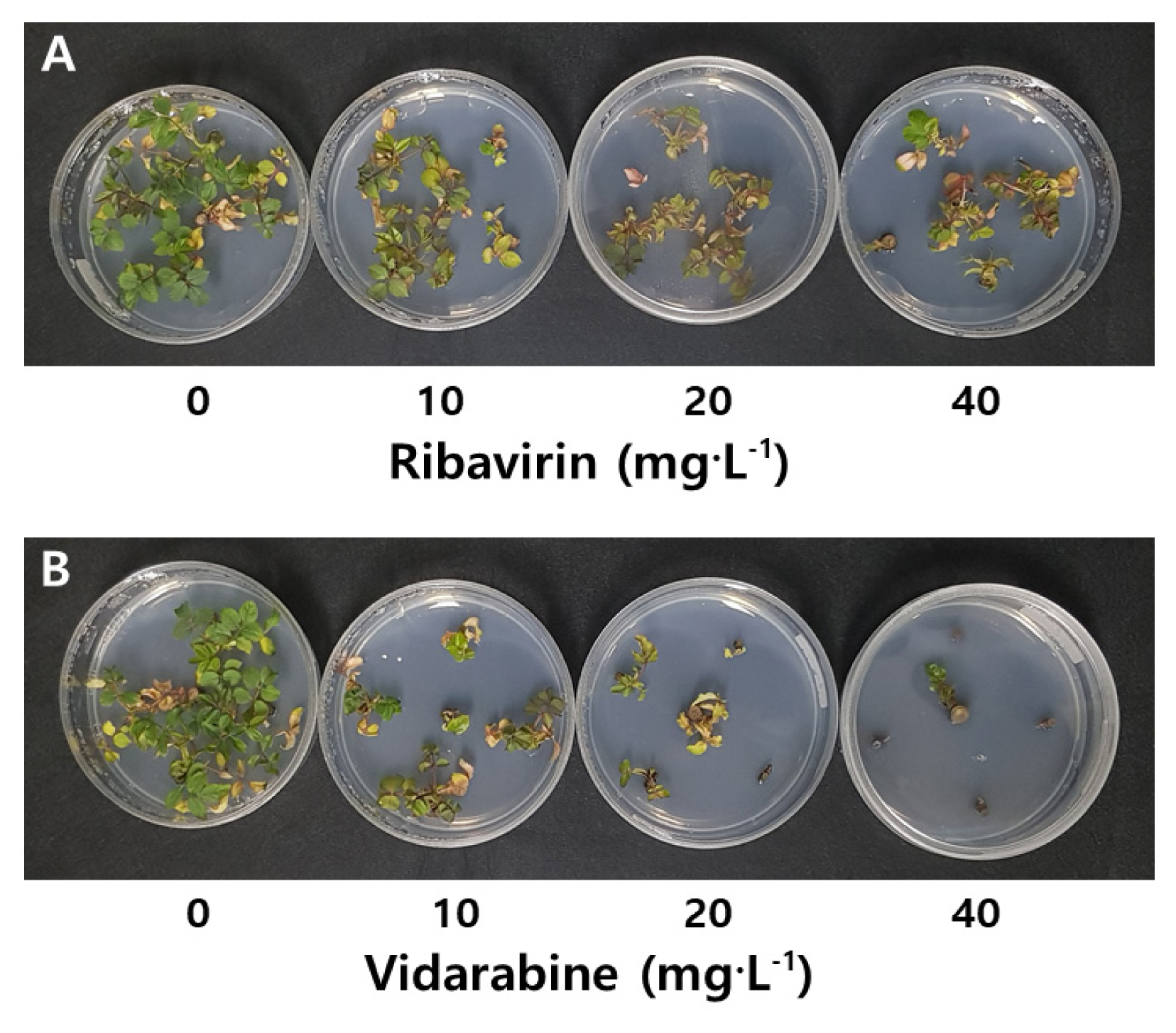
Shoot growth and virus elimination by antiviral agents treatment in rose 'Deep Purple'
Shoot growth and virus infection rate of rose 'Deep Purple' were examined after treatment with antiviral agents. Treatments of 10–20 mg · L−1 ribavirin were no statistical difference in shoot length and number of shoots and leaves compared to the control, and shoot growth was inhibited in 40 mg · L−1 treatment (Table 1). However, the survival rate was 93–100%, showing no difference between treatments. The infection rate of TRSV showed significant decrease in treatments of 20 and 40 mg · L−1 ribavirin, resulting in 25% and 50% respectively. And, the infection rate of ArMV was 50% in treatment of 10 mg · L−1 ribavirin, and no virus was detected in treatment of 20–40 mg · L−1, demonstrating the significant effect of the antiviral agent.

Shoot growth and virus infection rate of rose 'Deep Purple' by antiviral agent treatments 60 days after node culture
Cultures treated with vidarabine exhibited overall inhibition of growth compared to those treated with ribavirin, with higher concentrations leading to greater inhibition (Fig. 1). The survival rates for cultures with 20 and 40 mg · L−1 vidarabine were significantly low, resulting in 67% and 27%, respectively. The TRSV and ArMV infection rates of cultures treated with vidarabine were 80–100% and 67–100%, respectively, exhibiting no significant difference compared to the control. Therefore, it was judged that treatment of 20 mg · L−1 ribavirin was the most effective for shoot growth and virus elimination in node culture of 'Deep Purple'.
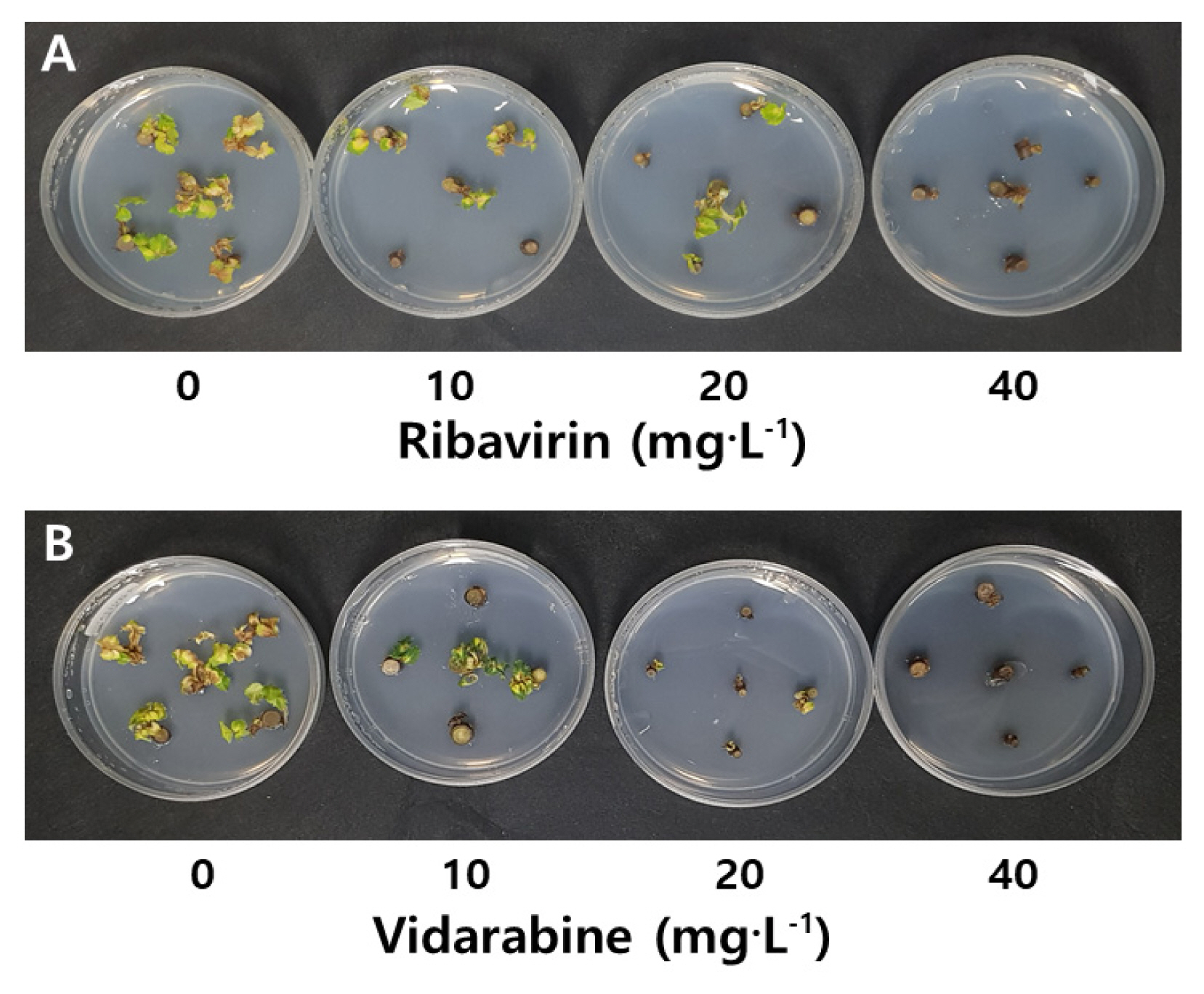
Shoot growth and virus elimination by antiviral agents treatment in rose 'Natal Briar'
Shoot growth and virus infection rate by treatments of antiviral agents ribavirin and vidarabine were examined in node culture of rose 'Natal Briar'. There was no significant difference in shoot length, number of shoots and leaves, and survival rate between control and 20 mg · L−1 ribavirin, but shoot growth and survival rate (7%) were significantly lowered at concentration of 40 mg · L−1 ribavirin (Table 2). And, TRSV and ArMV were not eliminated by ribavirin treatment.

Shoot growth and virus infection rate of rose 'Natal Briar' by antiviral agent treatments 60 days after node culture
In treatment of 10 mg · L−1 vidarabine, the shoot length and number of shoots/and leaves were significantly inhibited, and survival rate was 33.3% (Fig. 2). And, survival rate was 0% at 20–40 mg · L−1 vidarabine. However, the infection rates of both TRSV and ArMV were 50% in treatment of 10 mg · L−1 vidarabine, indicating the effectiveness of the agent in eliminating the viruses. In node culture of rose 'Natal Briar', ribavirin had no effect on virus elimination, whereas treatment of 10 mg · L−1 vidarabine was found to be effective in shoot growth and virus elimination.
Shoot growth and virus elimination by antiviral agents treatment in rose 'Pink Beauty'
In rose 'Pink Beauty', as the concentration of ribavirin increased, shoot growth was significantly suppressed compared to the control, and survival rates were significantly lowered to 53% and 13% at 20 and 40 mg · L−1 ribavirin, respectively (Table 3). And, it was observed that ribavirin had no effect on eliminating TRSV, whereas the infection rate of ArMV decreased to 33.3–66.7%, indicating that the agent had an effect on ArMV elimination.

Shoot growth and virus infection rate of rose 'Pink Beauty' by antiviral agent treatments 60 days after node culture
In the case of vidarabine, overall growth was significantly suppressed as the concentration increased, with a survival rate of 53% at 10 mg · L−1, 47% at 20 mg · L−1, and a drastic decrease to 0% at 40 mg · L−1 vidarabine (Fig. 3). The treatment of 10 mg · L−1 vidarabine exhibited no effect in eliminating both TRSV and ArMV, while treatment of 20 mg · L−1 showed a 50% infection rate, indicating that the agent had an effect on virus elimination. Thus, it was judges that treatment of 20 mg · L−1 vidarabine was the most effective in shoot growth and virus elimination in node culture of rose 'Pink Beauty'.
Discussion
The virus occurrences in floral crops cultivated in Korea were first reported in orchids by Lee and La (1976). In their report, cymbidium mosaic virus (CyMV) was detected in Cymbidium spp., Dendrobium spp., Cattleya spp., with an infection rate of 45% among the surveyed plants. Chang (1987) reported that through research on virus disease of ornamental plants in Korea, 11 kinds of viruses were found in carnations, gladioluses, amaryllises, irises, and orchids. In addition, Kim et al. (2011) reported on the virus disease occurrence among major floral crops in Korea, with reports on damages caused by chrysanthemum stunt viroid in chrysanthemums, odontoglossum ringspot virus (ORSV) and CyMV in orchids, carnation mottle virus in carnations, and cucumber mosaic virus (CMV) and tulip breaking virus in tulips. Among flowering trees cultivated in Korea, forsythia and hydrangea were first reported to have CMV infection in 1997 and 1972, respectively (Lee et al., 1982; Lee et al., 1997; Lee, 2004). In the case of roses, it was reported that domestically bred and imported varieties cultivated in Korea were infected with viruses such as TRSV, ArMV, and INSV (Roh and Yoo, 2023). This study also revealed that not only roses 'Deep Purple' and 'Pink Beauty' grown in Korea, but also rose 'Natal Briar' used widely in grafting propagation were infected with TRSV and ArMV.
Ribavirin is the most commonly used antiviral agent for virus elimination from plants. It is known that ribavirin is effective in eliminating CMV and cnidium vein yellowing virus-2 (CnYVY-2) in Cndium officinale, East African cassava mosaic virus (EACMV) in Manihot esculenta, and freesia mosaic virus (FreMV) and tobacco rattle virus (TRV) in Freesia hybrida. In addition, ribavirin treatment was the most effective in virus elimination at concentrations of 20–40 mg · L−1, and concentrations of 80 mg · L−1 and higher were found ineffective because of inhibited growth and reduced survival rate in plants (Choi et al., 2016; Kidulile et al., 2018; Kim and An, 2022). In this study, when 20 mg · L−1 ribavirin was treated in node culture of rose 'Deep Purple', high survival rate of 100% and reduced infection rate of TRSV and ArMV to 25% and 0% respectively, which was the most effective. In this study, when 20 mg · L−1 ribavirin was treated in node culture, rose 'Deep Purple' showed high survival rate of 100% and reduced infection rates of TRSV and ArMV to 25% and 0% respectively, which was the most effective.
In addition to ribavirin, other antiviral agents used include DHT, amantadine, vidarabine, acyclovir (ACV), and azidothymidine (AZT). However, the treatment effect of antiviral agents varies according to the species or variety of plants. For the lily symptomless virus (LSV) in Lilium Oriental hybrid 'Casa Blanca', the virus elimination rate was as high as 86–95% in treatment of 20–40 mg · L−1 ribavirin, whereas amantadine had no effect on virus elimination (Seo et al., 1999). Also, in the case of Solanum tuberosum, ACV and AZT had no effect in eliminating potato virus S (PVS) in tissue culture, whereas ribavirin was most effective in treatment (Park et al., 1994). On the other hand, in a study on elimination of GLRaV-3 (grapevine leafroll-associated virus 3) in Vitis vinifera 'Kyoho', when the media of shoot apical meristem culture were treated with antiviral agents, it was found that 20–40 mg · L−1 DHT and amantadine were more effective than ribavirin in virus elimination (Kim et al., 2003). There are few studies on the effect of antiviral agent vidarabine on virus elimination in plants. In the shoot-tip culture of Cybidium 'Golden Gate', it was reported that the effective concentration for inactivating PRSV and CyMV was 50–100 mg · L−1 vidarabine (Paek et al., 1996). In the case of rose 'Natal Briar' in this study, ribavirin showed no effect with 100% infection rate of TRSV and ArMV, whereas treatment of 10 mg · L−1 vidarabine was effective in lowering the infection rate (50%) of TRSV and ArMV. For rose 'Pink Beauty', treatment of 20 mg · L−1 ribavirin showed the infection rate of 100% for TRSV and 67% for ArMV, whereas treatment of 20 mg · L−1 vidarabine showed a 50% infection rate for both viruses, indicating that vidarabine was more effective in virus elimination than ribavirin. As such, the effects of antiviral agents varied according to the rose varieties, and the effective concentration for virus elimination also differed. In addition, vidarabine exhibited higher toxicity compared to ribavirin, which showed a greatly lower survival rate at concentrations of 20–40 mg · L−1. Therefore, it is judged that further studies need to be conducted at concentrations of 5–15 mg · L−1.
In this study, by treating virus-infected roses with antiviral agents, the infection rates were reduced to 25–50% for TRSV and 0–50% for ArMV, but were not completely eliminated. In order to completely eliminate the virus infected in plant, it is often treated by mixing methods such as chemical and heat treatment or meristem culture. It was reported that Cnidium officinale treated with 20 mg · L−1 ribavirin showed a 20.4% infection rate for ASGV, but when combined with heat treatment of 29°C, resulted in a 0% infection rate (Kim and An, 2022). In the case of Vitis vinifera, the combination of antiviral treatment of 20 mg · L−1 ribavirin and heat treatment at 37°C during shoottip culture exhibited a virus elimination rate from 84% to 100%, allowing the production of virus-free plants (Hu et al., 2021). Therefore, even in the case of roses, if antiviral agents treatment and heat treatment are combined during tissue cultures, it will be more effective in virus elimination, and it was judged that additional research is needed.
Conclusion
The plants of three cultivars infected with TRSV and ArMV were treated with ribavirin and vidarabine during node cultures to observe shoot growth and virus infection rate. In rose 'Deep Purple', vidarabine had no effect on virus elimination. On the other hand, treatment of 10–20 mg · L−1 ribavirin were no statistical significance in shoot growth and survival rate compared to the control. And, the infection rates of TRSV and ArMV were greatly reduced to 25% and 0% in treatment of 20 mg · L−1 ribavirin, respectively, demonstrating the effectiveness of ribavirin in virus elimination. In rose 'Natal Briar', treatment of 10–20 mg · L−1 ribavirin had a 100% infection rate of both TRSV and ArMV, indicating no effect on virus elimination. On the other hand, treatment of 10 mg · L−1 vidarabine greatly inhibited shoot growth and showed a low survival rate of 33.3%. However, both TRSV and ArMV infection rates were 50%, indicating effectiveness in virus elimination. In rose 'Pink Beauty', as the treatment concentration of ribavirin and vidarabine increased, the shoot growth and was survival rate was greatly reduced. Although ribavirin had no effect in TRSV elimination, the infection rate of ArMV was lowered to 33.3–66.7% in treatment of 10–20 mg · L−1 ribavirin. In treatment of 20 mg · L−1 vidarabine, the infection rates for both TRSV and ArMV were 50%, which was effective in eliminating the viruses. Thus, the effectiveness in virus elimination was different depending on the type and concentration of antiviral agents for each rose cultivar. And in node cultures of rose, treatment of 20 mg · L−1 ribavirin for rose 'Deep Purple', 10 mg · L−1 vidarabine for rose 'Natal Briar', and 20 mg · L−1 vidarabine for 'Pink Beauty' were most effective for virus elimination.