Comparison of Growth and Leaf Color Quality of Mesembryanthemum cordifolium f. variegata as Affected by Shading Levels
Article information
Abstract
Background and objective
Mesembryanthemum cordifolium is a plant belonging to the Aizoaceae family and is native to South Africa and Namibia. As a CAM plant, M. cordifolium exhibits strong resistance to drought stress and can be utilized for the removal of soil salinity and heavy metals. Furthermore, it is an important medicinal crop with anti-inflammatory and antidepressant effects. However, despite its potential benefits, research on the optimal growth environment for M. cordifolium remains scarce.
Methods
Therefore, this study selected M. cordifolium f. variegata with high ornamental value as the experimental plant and compared the effects of different shading levels on the growth and leaf color quality of variegated baby sun rose (M. cordifolium f. variegata). Six shading levels (0, 35, 45, 60, 75, and 99%, respectively) were designed using polyethylene (PE) shading films.
Results
The results showed that the shoot height, shoot width, dry weight, and chlorophyll content (SPAD units) were highest under the 60% shading level, while leaf length, leaf width, and fresh weight were highest under the 75% shading level. On the other hand, growth was relatively lower under the 0% shading level, which suggests that M. cordifolium f. variegata prefers shade over direct sunlight. On the contrary, M. cordifolium f. variegata showed growth even under the 99% shading level, proving to have strong shade tolerance, but the leaf color quality was lowerst.
Conclusion
In conclusion, to significantly increase the size and biomass of plants and improve leaf color quality when cultivating M. cordifolium f. variegata under shading treatment, it is recommended to cultivate it under 60–75% shading levels.
Introduction
Mesembryanthemum cordifolium is a kind of succulent plant that belongs to the family of Aizoaceae and is generally known as Aptenia cordifolia. M. cordifolium is native to South Africa and Namibia (Herppich and Peckmann, 1997), but it is also classified as an invasive species that has invaded an extensive range of Europe (such as Spain, Portugal, and Croatia), North Africa (such as Egypt and Tunisia), and South Asia (such as India), and it is also known to have extremely strong vitality and reproductive capacity (El Hawary et al., 2020; El Mokni et al., 2020; Ferrer Merino and Donat-Torres, 2011; Milovic et al., 2016; Muthulakshmipechiammal and Rajendran, 2018; Smith et al., 2019). M. cordifolium is capable of CAM photosynthesis (Cela et al., 2009; Peckmann and Herppich, 1998) and is highly resistant to drought stress (Fleta-Soriano et al., 2015; Pinto-Marijuan et al., 2017). M. cordifolium is also known to have strong salinity tolerance (Cela and Munne-Bosch, 2012; Karakas et al., 2020) as well as a beautiful shape so that it can be used as a coastal ground cover plant in certain regions. In addition, M. cordifolium is effective in removing salt from soil (Galecio Mio and Tarazona Dionicio, 2022) as well as heavy metals (Erdogan et al., 2011; Zaimoglu et al., 2009), and can also be used to noise reduction (Ccepaya Loayza, 2018; Delgadillo Valdez, 2018). In the pharmacological aspect, M. cordifolium has an anti-inflammatory effect (Waweru et al., 2017), and antidepressant components were found in the root extract (Said et al., 2021), which suggested its sufficient potential as a medicinal crop. However, despite the usefulness of M. cordifolium, the optimal environment for efficient cultivation is unknown, which is why related research is needed.
It is necessary to control abiotic stress, which is a negative factor for growth, for mass production of plants. Since producing healthy plants is directly related to the income of farmers, it is something to be fully considered. One of the simplest methods for controlling abiotic stress is to provide appropriate shading levels to each species. It has been reported that canopy shades formed by plants in nature have a positive effect on the survival rate and growth of plants growing in the lower part (Callaway, 1992; Greenlee and Callaway, 1996; Baumeister and Callaway, 2006). A previous study reported that shading can protect plants from mechanical damage, heat stress, and photoinhibition (Semchenko et al., 2012). However, on the contrary, excessively high shading levels may have a relatively negative effect on plant growth, which is why research is necessary on suitable shading levels for each species. Shading can protect plants and help with stable environmental control, and artificial shading can be mostly created using shading films or shading nets (Lee and Nam, 2022). Previous studies were conducted on the growth of succulent plants according to shading levels (Lee et al., 2021b; 2022c; Nam et al., 2022), the effect of shading treatment on the growth of ornamental flower crops (Jang et al., 2022; Kwon et al., 2020; Park and Kim, 2021), and adequate shading levels for vegetables and tea tree (Cao et al., 2022; Shim and Jeon, 2022; Sim et al., 2021). As such, studies reported different results for various plant species, and it is also necessary to investigate the most suitable shading level for the cultivation of M. cordifolium f. variegata.
Accordingly, this study analyzed the effects of different shading levels on the growth and leaf color of M. cordifolium f. variegata and provided relevant fundamental data.
Research Methods
Selection of the plant material
Among the plant genetic resources at the National Agrobiodiversity Center at Rural Development Administration in South Korea, we selected variegated baby sun rose (Mesembryanthemum cordifolium f. variegata) (genetic resource number: IT345915) as the experimental plant. Prior to the study, we cut the stems, limiting the number of leaves to 5 leaves, with a size of 3 cm, to ensure consistent plant size. For the study, we used plants with axillary buds developed by at least 2 cm after one month.
Setting of the shading levels
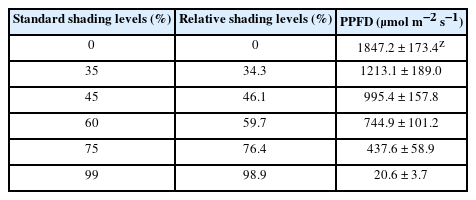
The study was conducted for a total of 8 weeks from May 2 to June 28, 2022, at the experimental greenhouse (37°38′40″ N 127°06′25″ E) in the Department of Environmental Horticulture, Sahmyook University. Plants were planted in round plastic pots with a diameter and height of 11 × 10.5 cm. We used the substrate for succulent plants by mixing decomposed granite (1–2 mm) with river sand (0.5–1 mm) and horticultural substrate (Hanareumsangto, Shinsung Mineral, South Korea) at a ratio of 6: 3: 1 (v/v/v) by volume, and planted each plant one by one at the center of the pot. We designed six levels of shading treatment: no shade (i.e., direct sunlight) (0%), greenhouse glass (35%), greenhouse glass and 1 layer of clear polyethylene (PE) shading film (45%), greenhouse glass and 1 layer of white PE shading film (60%), greenhouse glass and 2 layers of white PE shading film (75%), and greenhouse glass and 1 layer of black PE shading film (99%). For the photo-synthetic photon flux density (PPFD) of each shading level, we calculated the mean and standard deviation of the measurements taken at 1 p.m. once a week on sunny days using a portable spectral radiometer (SpectraPen Mini, Photon Systems Instruments, Czech Republic) (Table 1).
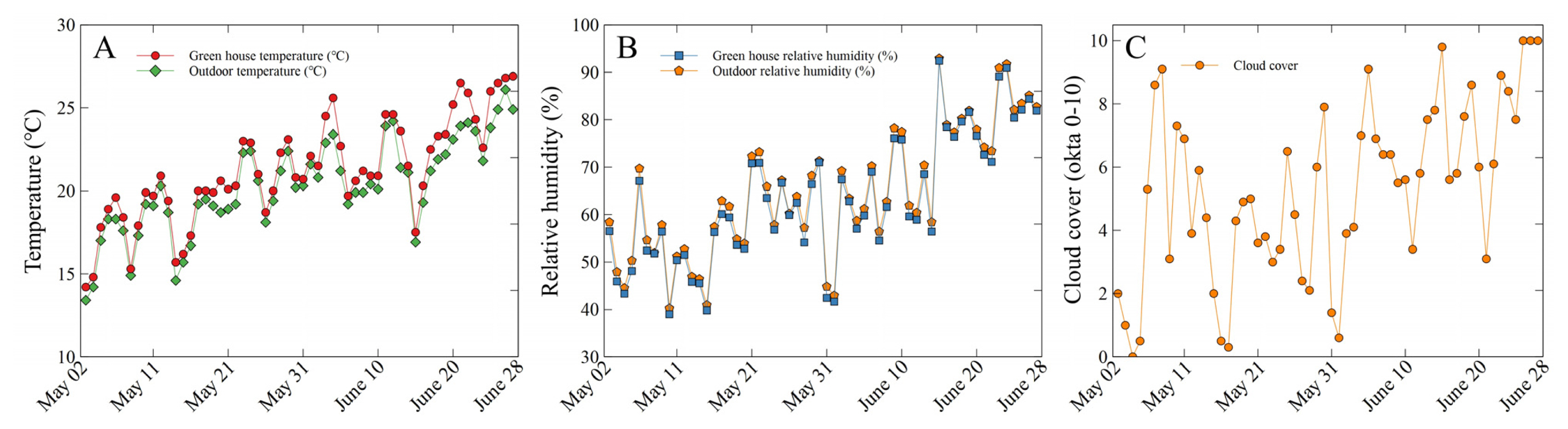
The plants of all treatments including the control (0% shading level) were arranged on beds that are 1 m high, and the average temperature in the greenhouse during the study was 21.2 ± 3.1°C, and the average temperature of the outdoor area where the control was placed was 20.1 ± 2.8°C (Fig. 1A). Here, the greenhouse facility where the study was conducted was ventilated by fully opening the top and sides for 24 hours. The relative humidity in the greenhouse was 63.2 ± 13.5%, and the relative humidity of the outdoor area was 64.6 ± 13.2% (Fig. 1B). The average cloud cover during the study was 5.2 ± 2.7 okta, and this was evaluated by visual observation, which is the same as the measurement method of Korea Meteorological Administration, at 1 p.m. every day (Fig. 1C). The plants were watered twice a week. In this study, watering continued until gravitational water drainage occurred.
Parameters and measurement methods
To investigate the effects of shading levels on the growth and leaf color of M. cordifolium f. variegata, various parameters were examined, including shoot height, shoot width, root length, leaf length, leaf width, fresh weight, dry weight, plant moisture content, chlorophyll content (SPAD units), and leaf color. The CIELAB color space, developed by the International Commission on Illumination in 1976, was utilized for assessing leaf color. The color coordinates, represented by L* (lightness), a* (green-red opponent colors), and b* (blue-yellow opponent colors) values, as well as ΔE*ab and RHS values, were analyzed. The shoot height was measured as the vertical distance from the ground to the highest point of the plant, and the shoot width by measuring the broadest area of the plant seen from above. For root length, we measured the longest of the plant roots. Secondary analysis was conducted on the SH/RL ratio, which represents the ratio of shoot height to root length, and the LL/LW ratio, which represents the ratio of leaf length to leaf width. Fresh weight was measured after washing the plant and naturally drying it for 24 hours in an enclosed space. Dry weight was measured after hot air drying of the plants for 24 hours at 85°C using a heat dryer (HK-DO135F, HANKUK S&I, South Korea). Here, we performed a secondary analysis by contrasting fresh weight with dry weight to analyze the plant moisture content, and equation (1) is as follows.
(x is plant moisture content, A is fresh weight, and B is dry weight)
SPAD units were measured using a portable chlorophyll meter (SPAD-502, Konica Minolta, Japan). CIELAB values were measured by setting the spectrophotometer (CM-2600d, Konica Minolta, Japan) to CIELAB D65/10° with reference to the leaf color measurement method by Lee et al. (2022a), after which we collected data including specular components. Here, to determine SPAD units and CIELAB, we randomly selected 3 leaves from each plant and measured the central part of the leaves. To compare the leaf color differences of M. cordifolium f. variegata at each shading level, we calculated the color differences by setting CIE76 ΔE*ab with reference to each shading level, and the equation (2) about the CIE76 color differences used is as follows (CIE, 2004).
(In this study, ΔE*ab ≤ 1.5 is regarded as ‘no color difference’ or ‘subtle color difference’, 1.6–3.0 as ‘very small color difference’, 3.1–6.0 as ‘small color difference’, 6.1–9.0 as ‘color difference’, 9.1–12.0 as ‘big color difference’, and ≥ 12.1 as ‘very big color difference’ or ‘completely different color’)
For RHS values, we compared the results of each of L*, a*, and b* with the Royal Horticultural Society (RHS) colour charts edition V and evaluated the leaf colors by selecting 2 approximate RHS values for each treatment. For leaf colors, we converted the means of CIELAB L*, a*, and b* to converted colors using Converting Colors developed by Zettl (2023).
Statistical analysis
Analysis of variance (ANOVA) was conducted using SAS 9.4 (SAS Institute, USA) to analyze the results of the study. To compare the means, statistical analysis was conducted using Duncan’s multiple range test at p < .05. The study was in a completely randomized design, with 5 plants assigned to each treatment in 3 replications.
Results and Discussion
Changes in plant growth
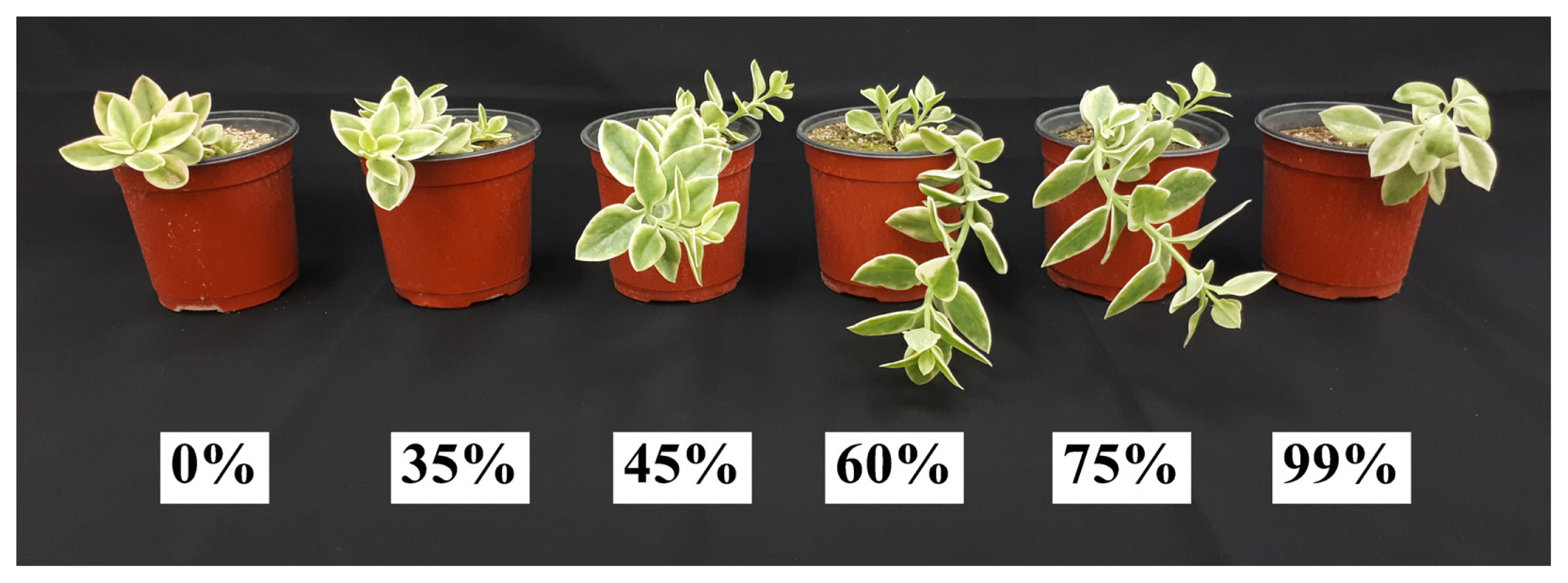
The plant sizes of Mesembryanthemum cordifolium f. variegata affected by the shading levels showed various differences (Fig. 2). The results showed that shoot height was tallest at 8.28cm under the 60% shading level (Table 2). This is similar to the results of previous shading study on Sedum zokuriense (Lee et al., 2021b) and Hylotelephium sieboldii cv. Mediovariegatum (Nam et al., 2022) discovered that shoot height was highest under the 65, 60% shading levels, respectively. Phenotypic plasticity is the ability of a plant to change its morphological characteristics so that it can adapt to the changing growth environment (Bradshaw, 1965; DeWitt et al., 1998; Sultan, 1987). The change in plasticity shown in shades is referred to as shade-induced phenotype (Weijschede et al., 2006). Here, in a shade-induced phenotype, we can expect to see an increase in the plant’s stems, shoot width, cover area, and leaf area (Lee and Nam, 2022). For shoot width of M. cordifolium f. variegata, the result of the 60, 75% shading levels in Duncan’s multiple range test showed the same significance level with 26.22 and 25.70 cm, respectively. Here, M. cordifolium f. variegata showed relatively low shoot width under 0, 35, and 99% shading levels compared to other treatments. Under the 0% shading level, factors such as light stress (Demmig-Adams and Adams, 1992), temperature rise, and water loss from soil may have had an effect. Additional research may be needed about light stress and water loss from soil by direct sunlight. On the contrary, under the 99% shading level, the growth rate may have decreased significantly because carbon dioxide assimilation is difficult, and the plant showed a shade avoidance response, implying that the amount of light received was extremely insufficient to overcome the low-level light environment. A previous study showed that Echeveria agavoides had relatively higher shoot height and shoot width in partial shading and shading compared to no shade, showing similar results as this study (Cabahug et al., 2017). Root length was longest under the 99% shading level at 21.26 cm. Some studies reported that the biomass allocated to the roots of Betula pendula was reduced under shading conditions (Van Hees and Clerkx, 2003), and Quercus robur and Fagus sylvatica also showed root growth inhibition under shading conditions (Van Hees, 1997). However, this study proved that shading treatment did not have a negative effect on the root length of M. cordifolium f. variegata, showing contrary results to the aforementioned studies. The SH/RL ratio that represents the ration of shoot height and root length was highest at 0.44 under the 60% shading level. Therefore, when cultivating M. cordifolium f. variegata under adequate shading level, shoot growth tends to be promoted relatively more than root growth.
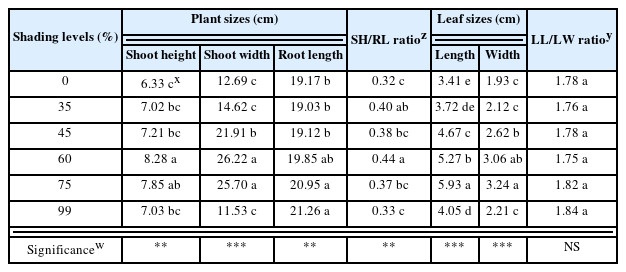
Plant sizes, ratio indices, and leaf sizes of Mesembryanthemum cordifolium f. variegata as affected by shading levels for 8 weeks
Leaf length was longest at 5.93 cm under the 75% shading level, and leaf width was also widest at 3.24 cm under the 75% shading level, indicating that we can expect the most increase in leaf area through shade avoidance response under the 75% shading level when cultivating M. cordifolium f. variegata. This is similar to the results of previous study where the leaf length and leaf width of H. telephium cv. Lajos was highest under the 75% shading level (Nam et al., 2022). Meanwhile, there was no significant difference in the LL/LW ratio that represents the ratio of leaf length to leaf width.
The fresh weight of M. cordifolium f. variegata was relatively higher under the 45, 60, 75% shading levels at the same significance level compared to other treatments as a result of statistical analysis, each showing 38.65, 38.61, and 41.33 g (Fig. 3A). Meanwhile, dry weight was highest under the 45, 60% shading levels at the same significance level, each showing 2.16, 2.20 g (Fig. 3B). Plant moisture content was highest under the 75% shading level at 95.1% (Fig. 3C). Considering these results, the 75% shading level seems to have increased the cell size of M. cordifolium f. variegata compared to other shading levels, and thus the plants could contain high moisture content. In summary of growth parameters, shading films prevented the abiotic stress of plants and had a positive effect on plant growth. In particular, better growth was shown in shading compared to growing M. cordifolium f. variegata under direct sunlight, indicating that it is better to use shades for the mass production of plants. Therefore, it is recommended to cultivate M. cordifolium f. variegata in about 60–75% shading levels in order to significantly increase the plant sizes and biomass.
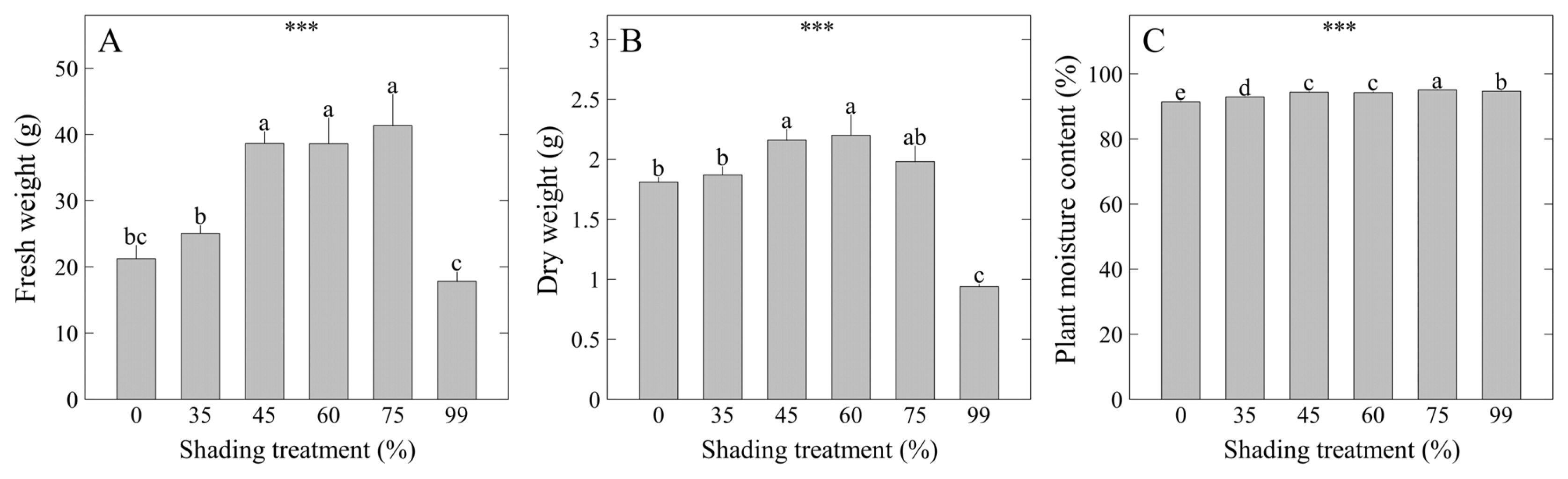
Plant weight and moisture content of Mesembryanthemum cordifolium f. variegata as affected by shading levels for 8 weeks: A) plant fresh weight; B) plant dry weight; C) plant moisture content. Vertical bars indicate the mean ± standard error (SE), and asterisks (***) indicate significant at p < .001. Different lowercase letters indicate significant differences at p < .05 based on Duncan’s multiple range test (DMRT).
Changes in leaf color and color differences
Chlorophyll content (SPAD units) of M. cordifolium f. variegata were highest at 22.87 under the 60% shading level, indicating that chlorophyll content was the highest in the same leaf area compared to other treatments (Fig. 4A). This result is similar to the results of shoot width and consistent with the reports of previous studies that S. zokuriense (Lee et al., 2021b), Hoya carnosa and Spathiphyllum wallisii (Lee et al., 2021a), and H. telephium cv. Lajos (Nam et al., 2022) showed an increase in chlorophyll content was observed concomitant with high shoot width under conditions of optimal shading level and light intensity. Therefore, creating a suitable growth environment for the plant species is an important factor to increase the shoot width and chlorophyll content of plants at the same time. Leaf color is a factor that enables intuitive evaluation of the external quality of plants and is important as it affects marketability (Lee and Nam, 2023). In previous studies, the increase in CIELAB L* that is the color space coordinate representing lightness showed a negative correlation with the growth parameters of a few succulent species (Lee et al., 2022b; 2022c), and L* was highest at 55.39 under the 99% shading level where the growth was lowest, thereby showing consistent results with previous studies (Fig. 4B). CIELAB a* that represents green-red opponent colors was highest at −6.24 under the 0% shading level, indicating that leaves were being colored significantly under direct sunlight (Fig. 4C). Moreover, CIELAB b* that represents blue-yellow opponent colors was highest at 21.89 under the 35% shading level (Fig. 4D). Meanwhile, some studies showed that L* and b* had a positive correlation (Kim et al., 2022; Lee et al., 2022b, 2022c), and similar trends were found under the 35, 99% shading levels.
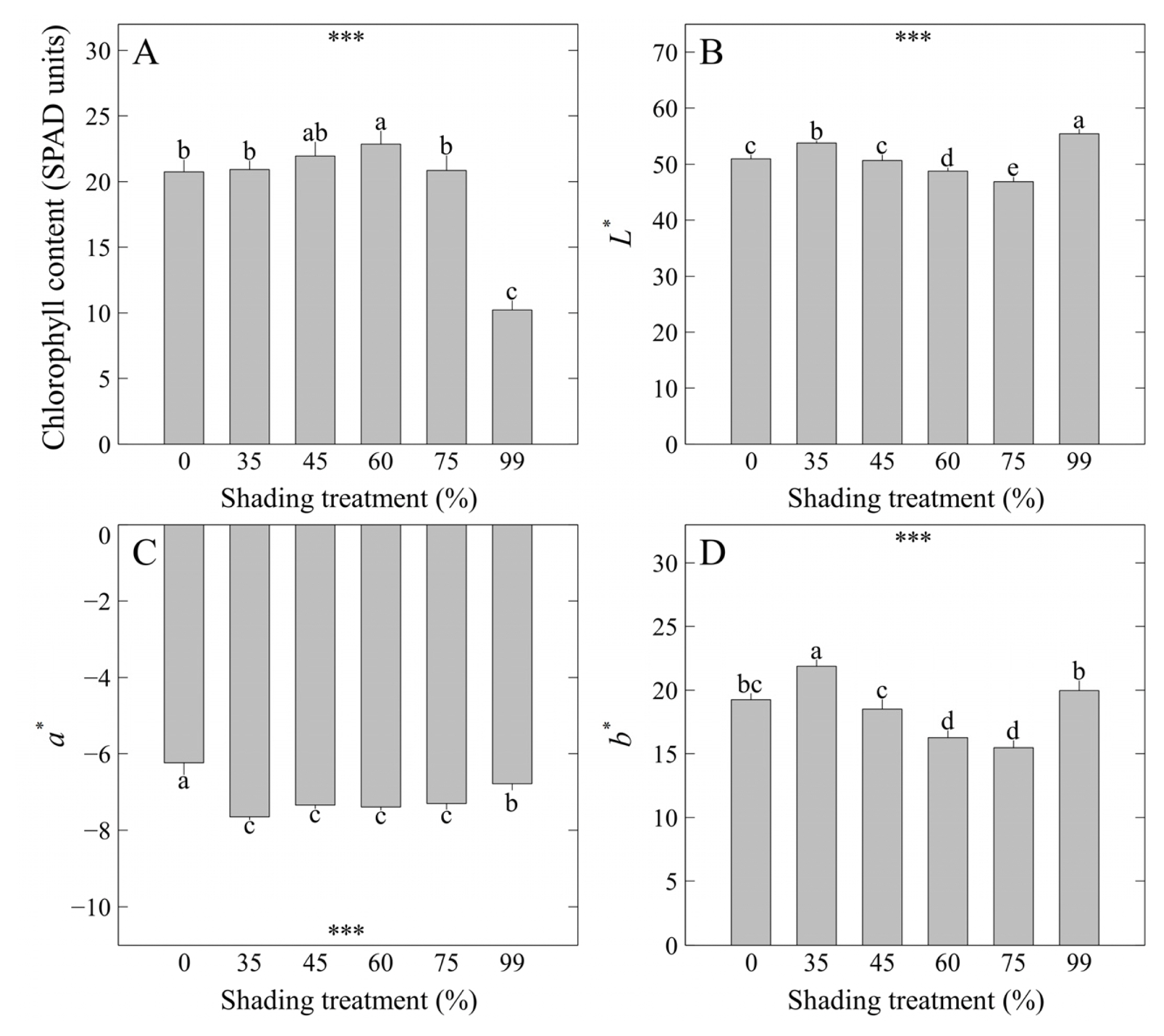
Chlorophyll content (SPAD units) and CIELAB values (L*, a*, and b*) of M. cordifolium f. variegata as affected by shading levels for 8 weeks: A) chlorophyll content; B) CIELAB L* value; C) CIELAB a* value; D) CIELAB b* value. Vertical bars indicate the mean ± SE, and asterisks (***) indicate significant at p < .001. Different lowercase letters indicate significant differences at p < .05 based on DMRT.
Leaf color difference analyzed with CIE76 (ΔE*ab) ranged from 1.35 to 9.64 by shading levels (Table 3). As for Royal Horticulture Society (RHS) values, the leaf colors of M. cordifolium f. variegata were N137D, 147B under the 60, 75% shading levels, indicating that they were closest to green, whereas the leaf colors in other treatments were 147B, 147C, 148B, indicating that they were relatively closer to yellow. Here, the leaf color difference under the 75% shading level showing the vigorous growth state and the 99% shading level showing the lowest growth state was highest at ΔE*ab = 9.64, indicating that there was ‘big color difference’.

Calculated of leaf color differences of CIE76 (ΔE*ab), RHS values, and converted color of M. cordifolium f. variegata grown under shading levels for 8 weeks
As a result of comprehensively evaluating the growth parameters, changes in leaf colors, and color differences, it was found that M. cordifolium f. variegata preferred the 60–75% shading levels compared to direct sunlight. Meanwhile, M. cordifolium f. variegata seems to have good shade tolerance so that it can show some growth under even the 99% shading level, but the SPAD units was the lowest and the lightness of leaves was the highest, thereby showing the low ornamental value of leaves. Accordingly, it is recommended to cultivate M. cordifolium f. variegata under 60–75% shading levels to significantly increase the plant sizes, biomass and improve the external quality of leaf colors.
Conclusion
Mesembryanthemum cordifolium is a succulent plant that belongs to the family of Aizoaceae and is native to South Africa and Namibia. M. cordifolium is a CAM plant that is highly resistant to drought stress and can be used to remove salt and heavy metals from soil, and it is also a medicinal crop with anti-inflammatory and antidepressant effects. However, despite its usefulness, research on the optimal growth environment is insufficient. Accordingly, this study selected variegated baby sun rose (M. cordifolium f. variegata) with excellent ornamental value as the experimental plant and compared the effects of shading levels on the growth and leaf color quality of M. cordifolium f. variegata. We designed six shading levels such as 0, 35, 45, 60, 75, and 99% using polyethylene (PE) shading films. The results showed that shoot height, shoot width, dry weight, and chlorophyll content (SPAD units) were highest under the 60% shading level, and leaf length, leaf width, and fresh weight were highest under the 75% shading level. Meanwhile, growth was relatively lower under the 0% shading level with the highest light intensity, indicating that M. cordifolium f. variegata prefers shades over direct sunlight. On the contrary, M. cordifolium f. variegata showed growth even under the 99% shading level, proving to have strong shade tolerance, but the leaf color quality was lower. In conclusion, it is recommended to cultivate M. cordifolium f. variegata under the 60–75% shading levels to significantly increase the size and biomass of plants and improve leaf color quality.