Effects of Phytoncide Extracts on Antibacterial Activity, Immune Responses, and Stress in Dogs
Article information
Abstract
Background and objective
Phytoncides are volatile and antibacterial organic compounds emitted by plants. In humans, these compounds are known to confer anti-bacterial, antioxidative, anti-inflammatory, immune-response-enhancing, and stress-reducing benefits. However, it remains largely unknown if phytoncides have parallel effects on dogs. Therefore, the aim of this study is to determine if phytoncides exhibit antibacterial activity, initiate an immune response, or alter stress hormone dynamics in dogs.
Methods
Phytoncide extracts used in the study were obtained from Hinoki cypress (Chamaecyparis obtuse) and Korean red pine (Pinus densiflora). To examine the effectiveness of the extracts at initiating and sustaining anti-microbial activity in dogs, we conducted a disc diffusion experiment on bacteria that cause skin diseases in the dog. Furthermore, we evaluated the effects of phytoncides on the immune response and stress hormone levels of dogs by spraying the phytoncide extract on the skin of the beagle twice a day for eight weeks.
Results
In this study, we investigated the effectiveness of phytoncides in eliminating bacteria cause skin infections in dogs. Our results confirm that mixed phytoncide extract was more effective than either phytoncide extract alone. After eight weeks, blood was collected from the common carotid artery of the beagle; we then measured the levels of immune response factors and cortisol. We found that with regards to the composition of white blood cells (WBCs), phytoncides significantly increased the proportion of lymphocytes and mononucleotides. They also increased the levels of immunoglobulin G and interferon-γ, while decreasing cortisol levels. Effects on several other immune markers were not significant.
Conclusion
We suggest that mixed phytoncide extracts have antibacterial effects in dogs. Additionally, these compounds seem to enhance immunity and reduce stress in dogs.
Introduction
There is a growing interest in forest bathing, which involves visiting forests for relaxation and recreation (Park et al., 2010). Forest bathing has been associated with a variety of therapeutic outcomes. For example, it has been shown to enhance immune responses, improve cardiovascular function, reduce anxiety, and relieve stress (Lee et al., 2019; Mao et al., 2012). Some of these effects are thought to be the resultants of phytoncide inhalation. Phytoncide inhalation in the forest environment restores immunological function and neuroendocrine hormone levels while counteracting the immunosuppression that stress causes (Chae et al., 2021).
Phytoncides are volatile organic compounds that are emitted by plants as a protective measure against harmful insects or herbivores (Duka and Ardelean, 2010; Shin and Lee, 2018). The term phytoncide is a combination of the Greek terms “phyton”, referring to plants, and “cide”, referring to killing, highlighting their antibacterial properties (Thangaleela et al., 2022). The particular phytoncide compounds vary across various tree species; however, almost all trees emit phytoncides (Das, 2020). The Hinoki cypress (Chamaecyparis obtusa) and Korean red pine (Pinus densiflora) produce particularly high levels of phytoncide (Kim et al., 2018; Lee et al., 2019). Phytoncides trigger an array of biological effects in humans. Specifically, these compounds have been shown to initiate anti-bacterial, anti-fungal, anti-stress, and anti-inflammatory effects across many contexts (e.g., forest bathing, aromatherapy, and holistic medicine (Kang et al., 2007; Kim et al., 2007; Woo and Lee, 2020). Several studies have shown that inhalation of phytoncides reduces stress and improves immune responses in humans (Li et al., 2012; Nam and Uhm, 2008). The beneficial effects of phytoncides are widely understood and well-investigated in humans, but the potential occurrence of these effects remains unconfirmed in dogs. Therefore, we aimed to investigate the antibacterial effects of two phytoncide extracts in bacteria. Additionally, we also aimed to study their biological effects on the immune and stress responses of dogs.
Research Methods
Extraction of phytoncide
Phytoncide was obtained by extracting essential oil from the leaves of C. obtusa and P. densiflora growing in South Korea.
The essential oil of plants is produced by various methods such as steam distillation, critical fluid extraction, expression, and solvent extraction, etc. Among them, steam distillation is known as the most widely used method of essential oil extraction (Božović et al., 2017; Masango, 2005).
In this study, C. Obtusa and P. densiflora essential oils were extracted using steam distillation method. Each essential oil was extracted through the following processes. After the C. Obtusa or P. densiflora needles being mixed with the distilled water and throwing in the steam distillation extraction device, it was heated at 100°C for 2 hours and the steam distillation was enforced. Then, the extracted essential oils were dehydrated over anhydrous sodium sulfate.
Mixed phytoncide was used by blending C. obtusa and P. densifola essential oils extracted by steam distillation at the same ratio.
Components of phytoncide
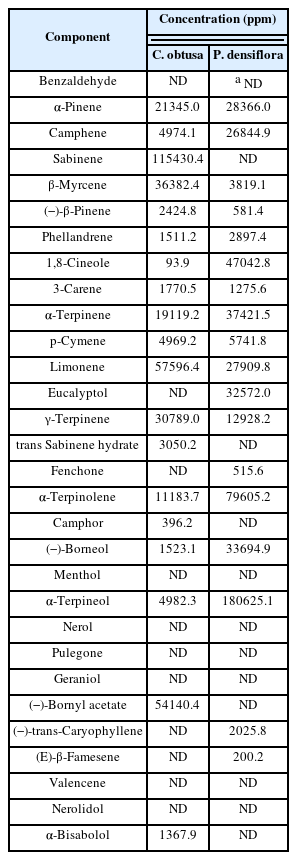
In order to analyze the phytoncide components of the extracted essential oils, a professional analysis agency (P CAM Korea, Daejeon, Korea) was requested. Table 1 shows the phytoncide components of C. Obtusa essential oil and P. densiflora essential oil. A total of 19 components, including Sabinene, Limonene, and Bornyl acetate, were detected in C. Obtusa essential oil, and a total of 18 components, including alpha-Terpineol, alpha-Terpinolene, and 1,8-Cineole, were detected in P. densiflora essential oil (Table 1).
Bacterial strains
The bacteria used to determine the antibacterial activities of different phytoncide extracts were as follows: Gram-positive: Staphylococcus aureus and S. taphylococcus intermedius; Gram-negative: Escherichia coli, Proteus mirabilis, and Pseudomonas aeruginosa. All bacterial strains were obtained from the culture collection of the Veterinary Medicine and Microbiology Department (Chungnam National University, Daejeon, Korea). Lysogeny broth (LB) agar plates were prepared by mixing 8 g of nutrient broth and 15 g of agar with 1 L of water, and the medium was autoclaved at 120°C for 30 min. All strains were grown on LB plates at 28°C and cultured at 37°C in LB medium.
Antibacterial activity assay
The antibacterial activities of P. densiflora and C. obtusa extracts were evaluated using a standard disc diffusion method. The phytoncide extract was incorporated into 6-mm-diameter sterile filter paper discs and dried. Three discs were placed on LB agar plates seeded with bacteria and incubated overnight at 37°C.
Animals study
All dogs (2–6 years of age) were obtained from the animal reproduction and physiology laboratory of the animal science and biotechnology department (Chung-Nam National University, Daejeon, Korea). This study used ten beagle dogs (2–6 years of age). Beagles were randomly divided into two groups (n = 5 per group). The skin of the beagles in the phytoncide group was sprayed twice a day for 8 weeks. The control group was treated with sterilized distilled water. After 8 weeks, blood was collected from the common carotid artery, centrifuged at 12,000 rpm for 15 min at 4°C, and serum was separated. The serum was separated and stored at −70°C until analysis. Serum was used to assess immune responses and cortisol levels. Body weight was recorded at weeks 0 and 8. All dogs were housed in an approved breeding facility provided by the Chung-Nam National University and were housed in an environment with temperature (22–23°C), humidity (50–60%), and light (12 h light) control. The dogs were provided with a continuous supply of water and fed twice a day.
Biochemical analysis
Blood was collected from the common carotid artery of beagles and centrifuged at 12,000 rpm for 15 minutes at 4°C. The separated serum was commissioned by DKKorea Inc. (Seoul, Korea) and evaluated for white blood cells (WBCs), neutrophils, lymphocytes, and cortisol using an XN-1000 analyzer (Sysmex, Kobe, Japan).
Assay of immune response and cortisol levels in serum by ELISA
The concentrations of immunoglobulin G (IgG), tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and interleukin (IL)-2 were assessed using an enzyme-linked immunosorbent assay (ELISA) kits following the manufacturer’s instructions (Abcam, Cambridge, UK). Absorbance readings were taken using a BioTek absorbance microplate reader (BioTek, Santa Clara, CA, USA).
Statistical analysis
All statistical analyses were performed using Sigma Plot software (version 12.0, Systat Software Inc., Chicago, IL, USA). Comparisons between groups were analyzed using the t-test, and the results are expressed as mean ± standard deviation (SD). Graphical representations of the results were made using GraphPad Prism software (version 5.0; GraphPad Software, Inc., San Diego, CA, USA). A probability p-value of less than 0.05 was considered statistically significant.
Results and Discussion
Effects of phytoncides on bacteria causing skin infections in dog
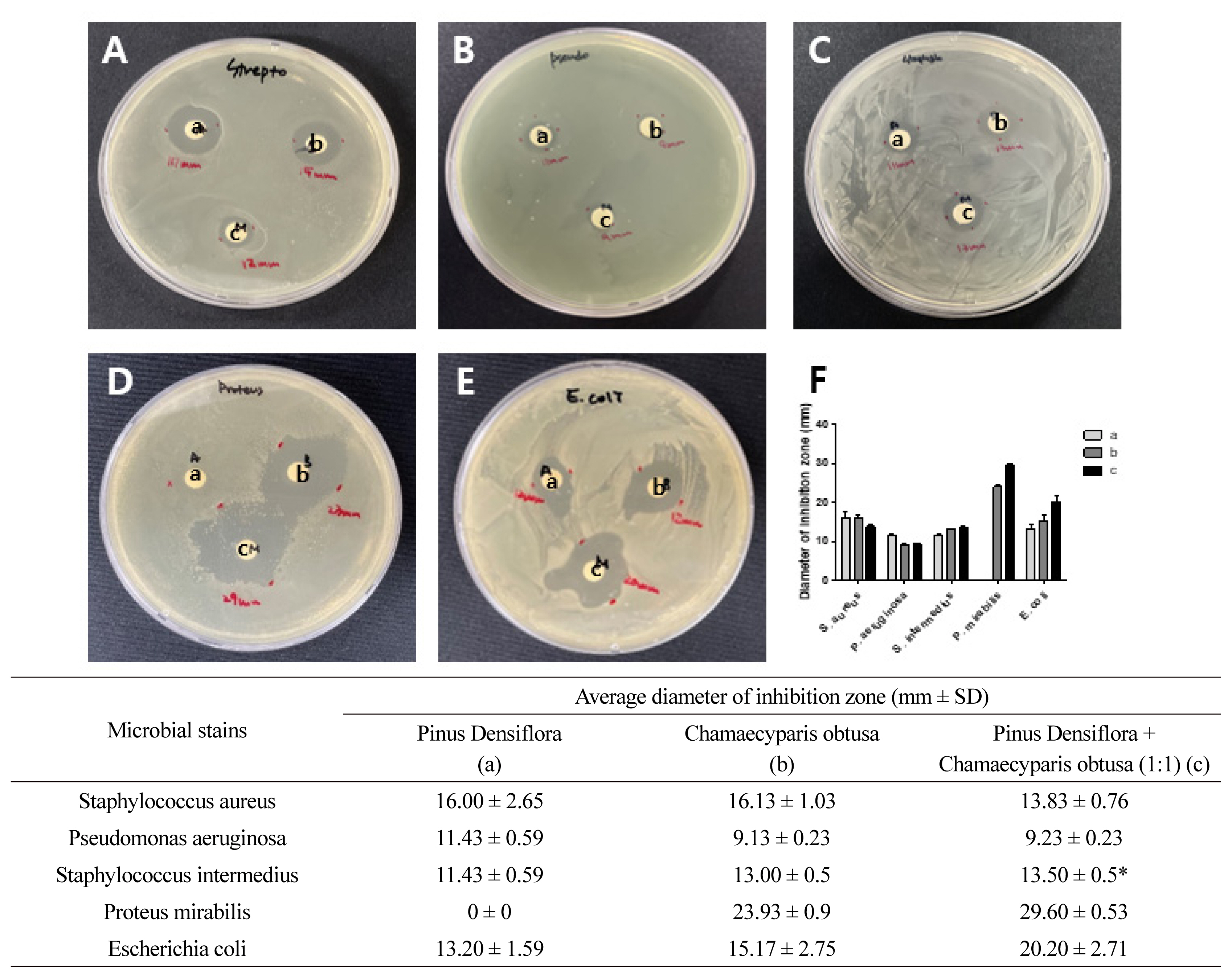
Phytoncides are known to exhibit antibacterial activity, hence we investigated the antibacterial activity of two phytoncide extracts against a representative bacteria known to cause skin diseases in dogs. It has been shown that phytoncides have an antibacterial effect on oral pathogenic bacteria (Kang et al., 2007). The phytoncide significantly enhanced the antibacterial activity of ampicillin against S. mutans and S. sobrinus. Similarly, phytoncides have an antibacterial effect on Porphyromonas gingivalis, one of the most important causative agents of periodontitis and halitosis (Kim et al., 2007). In evaluating the anti-bacterial activity of P. densiflora and C. obtusa phytoncides, we surprisingly found that anti-microbial activity was the highest in mixed phytoncide extracts, as compared to single-species extracts. The antibacterial activity of phytoncides was confirmed by measuring the diameter of the inhibition zone formed using the paper disc diffusion method when five types of bacteria that can cause skin infections in dogs were exposed to extracts (Fig. 1). The diameter of the inhibition zone was measured more than three times. Korean red pine phytoncide had high anti-bacterial activity in P. seudomonas aeruginosa (11.43 mm) than other phytoncides but no anti-bacterial activity in P. roteus mirabilis alone. Hinoki cypress phytoncide showed antibacterial activity in all bacteria and had high anti-bacterial activity in S. taphylococcus aureus (16.13 mm). However, mixed phytoncide from Korean red pine and Hinoki cypress showed evenly distributed antibacterial activity across all bacteria and high anti-bacterial activity in S. taphylococcus intermedius, P. roteus mirabilis, and E. scherichia coli (13.50 mm, 29.60 mm, and 20.20 mm, respectively). Based on these results, we conclude that the mixed Based on these results, we conclude that the mixed phytoncide extracts of Korean red pine and Hinoki cypress have a strong antibacterial effect against bacteria capable of causing skin infections in dogs. Additionally, these extracts seem to be particularly effective against S. taphylococcus intermedius, P. roteus mirabilis, and E. scherichia coli. These findings suggest that the anti-bacterial action of phytoncides extract from P. densiflora and C. obtusa can be effective in preventing and treating bacterial skin infections by inhibiting bacteria.
Effects of phytoncides on immune responses in dogs
Phytoncides affect immune response (Li et al., 2012). Previous studies have shown that phytoncides increase the number of natural killer (NK) cells, a type of cytotoxic lymphocyte, and improve immune responses (Kim et al., 2018; Li, 2010). Since inhalation of phytoncides has been shown to increase the number of NK cells in the body’s circulatory system and help reduce human stress, it has also been shown to be beneficial to humans (Li et al., 2006). Thus, we aimed to evaluate the effects of phytoncides on the immune system of dogs. To investigate the effect of phytoncides on the immune response of dogs, levels of WBC, IgG, IFN-γ, TNF-α, and IL-2 were determined using the serum.
The level of IgG was significantly heightened (approximately 2-fold) in the phytoncide group compared to that in the control group (Fig. 2A). The levels of TNF-α and IL-2 slightly increased in the phytoncide group compared to those in the control group, but the difference was not significant (Figs. 2C, 2D). However, IFN-γ levels were significantly increased (3- to 5-fold) in the phytoncide group compared to the control group (Fig. 2B). Treatment with mixed phytoncides of P. densiflora and C. obtusa was shown to be associated with increased the levels of WBCs, lymphocytes, IgG, and IFN-γ in dogs at 8 weeks compared to levels in the control group.
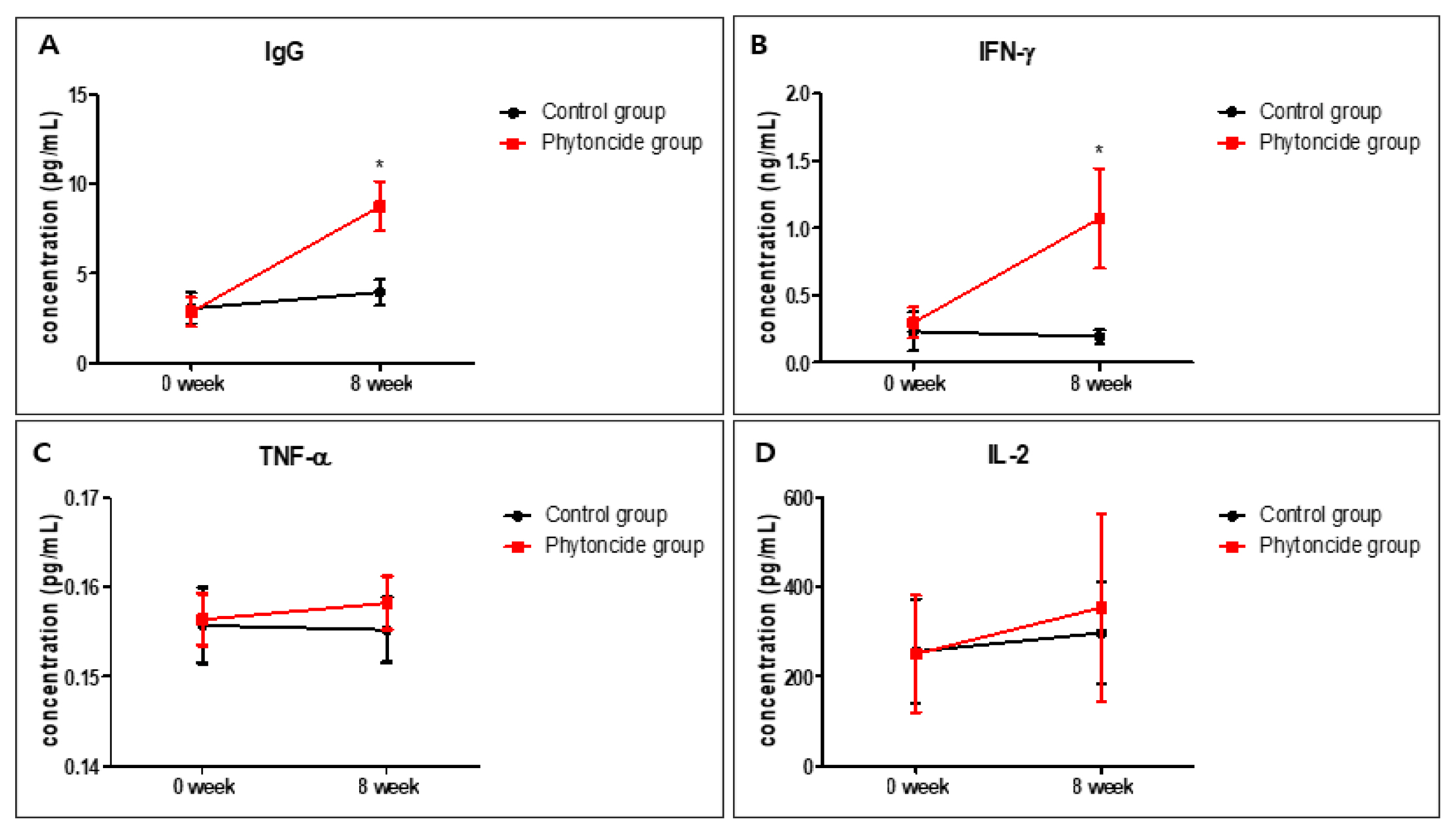
Effect of phytoncide on immune response in the dog. Effect of phytoncides on a stress hormone in dogs.
However, the body weight of dogs has remained constant (Table 2). We also demonstrated that phytoncide treatment significantly increased the proportion of lymphocytes and mononucleotides in WBC and decreased the proportion of granulocytes (Table 3).
WBCs prevent the establishment of an infection and therefore play an important role in the body (Honda et al., 2016). IgG modulates macrophage function and promotes immune response and IFN-γ, TNF-α, and IL-2 are cytokines responsible for immune responses and are produced by activated macrophages or lymphocytes (Anderson et al., 2002; Van der Meide and Schellekens, 1996). These act as important markers of the immune system. Our study indicated that phytoncide exposure may contribute to an improved immune response in dogs.
Effect of phytoncides on a stress hormone in dogs
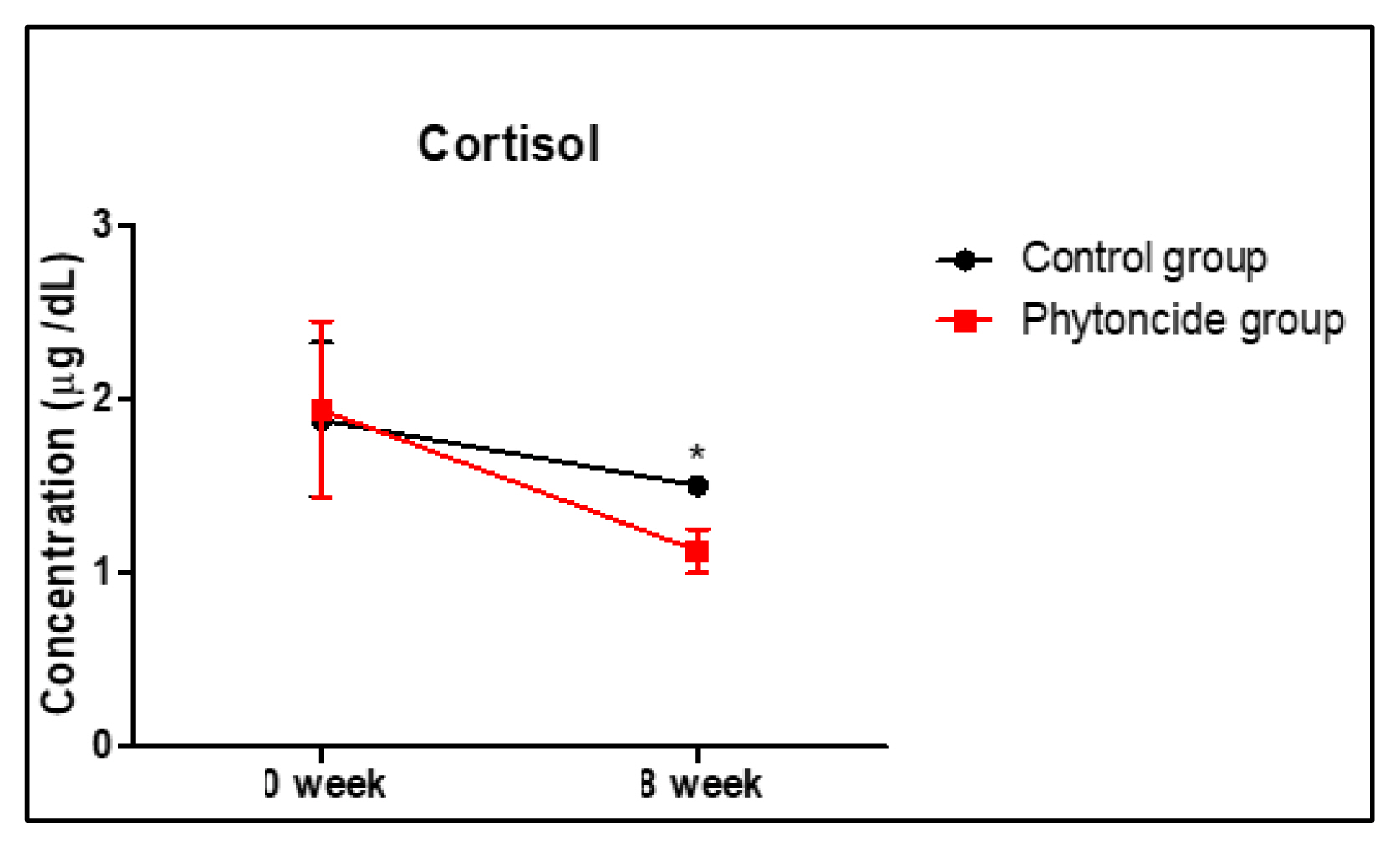
In humans, phytoncides are well-known for their stress-reducing qualities (Woo and Lee, 2020). A forest environment enriched with phytoncides suppresses sympathetic nervous activity, reduces blood pressure, reduces heart rate, and decreases the level of stress hormones such as cortisol (Antonelli et al., 2019; Farrow and Washburn, 2019; Ideno et al., 2017). Additionally, forest bathing in the presence of phytoncides promotes brain functioning by reducing mental stress (Wen et al., 2019). Previous studies have reported that the inhalation of phytoncide extract from pine trees and cypress trees is partially effective in alleviating stress in college students (Nam and Uhm, 2008). Cortisol is commonly known as a stress hormone and is recognized as a major indicator of stress responses (Payne and Nadel, 2004). Stress impairs immune responses and may promote the initiation and progression of disease (Cohen et al., 2007; Padgett and Glaser, 2003). During stress, cortisol secretion is increased through the increased activation of the hypothalamus-pituitary-adrenal system and sympathetic nervous system (Kudielka and Wüst, 2010). Cortisol plays a crucial role in body and brain function as it regulates numerous underlying processes, such as blood pressure, glucose metabolism, inflammation, and immune responses. Overall, cortisol allows organisms to flexibly adapt to various environmental challenges (Dedovic et al., 2009; Denson et al., 2009; Fraser et al., 1999; Khani and Tayek, 2001; Nijm and Jonasson, 2009). We investigated the effects of phytoncides on stress hormones in dogs. In the present study, we measured the concentration of cortisol in serum after exposure to phytoncides. We found that cortisol levels were significantly lowered (approximately 2-fold) in the phytoncide group compared to levels in the control group at 8 weeks (Fig. 3). These results suggest that phytoncide inhalation can help relieve stress in dogs.
Conclusion
Phytoncide is a volatile compound that emits by trees. In humans, the effect of phytoncide has various functions such as anti-bacterial, antioxidative, anti-inflammatory, immune- response-enhancing, and stress hormone-reducing benefits. However, these effects are not well-known in dogs. Therefore, we studied the antibacterial activity of phytoncide extract from P. densiflora and C. obtusa and their effects on immunity and stress in dogs.
This study shows that phytoncide extracts from P. densiflora and C. obtusa have anti-bacterial activity against bacteria that cause skin infections especially S. taphylococcus intermedius, P. roteus mirabilis, and E. scherichia coli. As the result, we can expect the effect of phytoncide extract to prevent skin infections caused by bacteria.
Furthermore, these compounds have also been shown to increase the number of WBC and levels of immunoglobulin G and IFN-γ, and decrease cortisol levels in dogs. A combination of phytoncides from P. densiflora and C. obtusa exerts immune-boosting and stress-reducing effects in beagles.
Consequently, we have shown that phytoncides emitted from plants have a good effect not only on humans but also on their companion animals, dogs. We suggest that a phytoncide-based combination treatment may be beneficial in animals more broadly, as well as in humans.
In future studies, the effects of phytoncides on diseases in dogs need to be studied further. We hope that the health and quality of life of humans and their companion animals can be enhanced by further studies into phytoncide efficacy.