Inhibitory Activity of Pseudomonas putida and Bacillus licheniformis Supernatants on PMMoV in Chili Pepper
Article information
Abstract
Background and objective
Plant viruses are major obstacles to enhancing crop productivity in both agriculture and horticulture throughout the world, resulting in losses of several billion dollars every year. Controlling viruses is arduous, so agrochemicals are widespread. To minimize the usage of those, this study’s objective was to assess bacterial cultures supernatants on pepper mild mottle virus (PMMoV) in chili pepper plants and identify its secondary metabolites.
Methods
This 48-h grown Pseudomonas putida (PP) and Bacillus licheniformis (BL) cultures supernatants were foliar sprayed separately in chili pepper plants 24-h before PMMoV inoculation (T1), and 24-h before and after PMMoV inoculation (T2), 2wpi (week’s post inoculation), the virus titer was determined by using a double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA), and the supernatants were extracted with ethyl acetate and concentrated by rota-evaporation before being analyzed in gas chromatography mass spectrometry (GC-MS).
Results
The culture supernatants of PP and BL inhibit PMMoV by 43–47% in both the treatments (T1 & T2) compared to the control. The GC-MS chromatogram of two cultures of supernatants identified the molecules of cyclo (Pro-Val), cyclo (Pro-Leu), and cyclo (Phe-Pro). Commercial forms of these three molecules at three concentrations showed a hypersensitive response, ranging from 45–65% for PMMoV in Nicotiana glutinosa.
Conclusion
The results revealed that supernatants of PP and BL-containing compounds have biological control of PMMoV in chili pepper plants.
Introduction
Chili pepper is a vegetable crop, susceptible to 13 different types of plant viruses throughout the world (Lockhart and Fischer, 1974; Wetter et al., 1984). Among these viruses, pepper mild mottle virus (PMMoV) has been reported as a great threat to pepper production (Jarret et al., 2008; Roberts and Adkin, 2001), due to mainly its highly contagious nature and long persistence in soil (Ikegashira et al., 2004). PMMoV is a non-enveloped, rigid, rod-shaped, single-stranded positive-sense RNA, which belongs to the genus Tobamovirus. On farms, PMMoV also remains viable for long periods in soils after infected crops have been removed or harvested. Composting and drying have been shown to only slightly reduce the detection of PMMoV but may impact the infectivity of the virus (Aguilar et al., 2010; Petrov, 2014). A recent report revealed that PMMoV remains infective even after wastewater treatment in wastewater treatment plants (WWTPs) (Bacnik et al., 2020). PMMoV is such a resilient virus that it can survive the harsh environment of the human gastrointestinal tract, and it remains infectious even after excretion in feces, where it is the most dominant virus, depending on nutritional habits (Zhang et al., 2006). This is why PMMoV has emerged as a link between waterborne tobamoviruses and enteric viruses. PMMoV is present worldwide in water matrices that come into contact with human fecal pollutants, from treated wastewaters to rivers and seawater, where it shows minimal resistance to changes in the environment (Kitajima et al., 2018; Symonds et al., 2018; Bivins et al., 2020). The irrigation of water to chili pepper plants, that are infected with PMMoV and exhibit symptoms like mottling and yellow/green mosaic in leaves, and small malformed, mottled fruit and resulting in loss of pepper yield (Jarret et al., 2008; Roberts and Adkin, 2001; Kim et al., 2012; Rialch et al., 2015). One previous study stated that interactions of PMMoV with the human immune system and suggested that the virus may cause clinical symptoms in humans, such as fever, abdominal pains, and pruritus, however, these symptoms may have been confounded by spicy food rich in peppers or pepper based products (Colson et al., 2010).
To date, agricultural practices are purely relied on the use of different kinds of agrochemicals to meet the maximum crop yield from the various abiotic and biotic factors to the oversized populations around the entire globe (Searchinger et al., 2018; United Nations Department of Economic and Social Affairs Population Division, 2022). Overpressure in the crop demand and agricultural expansion could result in pesticide use and fertilizer application increases approximately 10-fold and 2.7-fold respectively (Rohr et al., 2019). The excessive usage of agrochemicals poses a significant threat to living organisms and is also environmentally hazardous (Sponsler et al., 2019). While the development of genetically modified crops with disease resistance, enhancement the crop yield, and commercialization of the farmers are realistically tedious and lengthy processes (Kanchiswamy et al., 2015). Therefore; other alternative eco-friendly control strategies must be explored against chemical inputs or genetic modification of crops. Plant-beneficial bacteria and fungi, living in the soil as free organisms or as endophytes, that trigger plant growth and protect plants from diseases and abiotic factors have been well documented by several researchers (Tonelli et al., 2010; Radhakrishnan et al., 2014). The application of plant-beneficial microorganisms is an alternative to chemical fungicides, bactericides, and nematicides and also a sustainable approach for improving plant growth and controlling many plant diseases (Choudhary and Johri, 2009; Radhakrishnan et al., 2013; Adam et al., 2014; Egamberdieva et al., 2014; Kohl et al., 2019). Soil microorganisms produce a wide range of secondary metabolites enabling them to compete with neighborhood microorganisms (Brakhage and Schroeckh, 2011; Raaijmakers and Mazzola, 2012; Garbeva and Weisskopf, 2020). Most of the research on microbial secondary metabolites focuses on non-volatile compounds, increasing attention is paid to microbial volatile organic compounds (mVOCs). VOCs are low molecular weight (< 300 Da), high vapor pressure, low boiling points, and are lipophilic (Schulz-Bohm et al., 2017). VOCs are important for antibiosis and signaling for symbiotic interactions (Effmert et al., 2012). The capacity of mVOCs to suppress neighboring pathogens and signals to plants demonstrates their potential to be exploited as alternatives to chemical fertilizers and pesticides (Thomas et al., 2020) and also have negligible hazardous effects on animals and the environment (Tilocca et al., 2020). Among several species of plant growth-promoting bacteria/rhizobacteria (PGPB/PGPR), Pseudomonas and Bacillus spp. have been identified as the predominant communities (Kang et al., 2015), and a few of the PGPB have been commercialized due to their survival within a diverse range of biotic and abiotic environments. PGPRs can produce and induce a wide diversity of useful bioactive metabolites (Beneduzi et al., 2012). Specifically, Pseudomonas and Bacillus were important members of the protective microbiome Wei et al. (2019). The recent report on the bacterial volatile compound - 2, 3-butanediol induced the pepper resistance to multiple viruses in the laboratory and field level application (Kong et al., 2018). There is a piece of very limited information on the bacterial volatile compounds in the plant viruses, so this study aimed an assessment of Pseudomonas putida and Bacillus licheniformis supernatants on PMMoV in chili pepper and identification of active metabolites in the supernatants.
Research Methods
Site
This study was conducted in the greenhouse of the National Institute of Horticultural and Herbal Science (NIHHS), Rural Development Administration (RDA), Wanju-gun, Republic of Korea.
Seedlings
Cheongyang pepper (Capsicum annuum L.) and Nicotiana glutinosa seedlings were grown and cultured in the soil pot. These seedlings in good sizes were used for the experiment.
Virus source
PMMoV was maintained in N. tabacum leaves and confirmed virus viability by the immunostrip method. Infected leaves were collected aseptically and ground with 10 mM phosphate buffer (pH-7.0) using a sterilized plastic pouch in the ratio of 1 g leaf tissue per 10 mL of the buffer. The suspension is used as the source of inoculum for chili pepper and N. glutinosa infection. The first two true leaves of each chili pepper and good leaf size of N. glutinosa plants were lightly dusted with Carborundum (Thermo Fisher Scientific, Waltham, MA, USA) and then rubbed with inoculum from leaf base to tip, which contains PMMoV-infected tobacco leaf suspension. All the plants including the controls were inoculated with PMMoV.
Bacterial cultures
The bacterial cultures were obtained from the Rural Development Administration (RDA) gene bank, in the Republic of Korea. The cultures are Pseudomonas putida - (KACC-12538) and Bacillus licheniformis - (KACC NO-10307).
Composition of media and culture conditions
The Luria-Bertani (LB) broth contains the following ingredients (g·L−1) : Tryptone-10, Yeast extract -5, Sodium chloride -5, and pH was adjusted to 7.0. LB broth was used for the cultivation of PP and BL. It was purchased from BD DifcoTM (Thermo Fisher Scientific Inc., Sparks, MD, USA). For the growth of P. putida and B. licheniformis cultures, the autoclaved LB broth was inoculated separately with the individual bacterial cultures. The culture flasks were incubated in an orbital shaker at 175 rpm at 37°C for 48 hours. The 48-hour grown cultures was centrifuged for 10 min, at 4°C, at 10,000 xg to separate the bacterial cultures from the supernatant, which was then used for foliar application in chili pepper plants.
Foliar application
The supernatants of PP and BL were foliar sprayed separately in chili pepper plants 24-h before PMMoV inoculation (T1), and 24-h before and after PMMoV inoculation (T2), and the development of symptoms was observed for about 2-weeks. The young leaves were collected, and PMMoV accumulation was quantified by DAS-ELISA assay.
Double antibody sandwich-enzyme linked immunosorbent (DAS-ELISA) Assay
Double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) method (Gangireddygari et al., 2021) was used for the quantification of the virus as per the manufacturer’s protocol (Agdia, Elkhart, IN, USA). The experimental setup of chili pepper seedling’s upper leaf sample was collected at 2wpi along with control samples and extracts of the PMMoV antigen by using a general extraction buffer. The extracted samples of (100 μL) were placed in the capture-antibody coated ELISA plate and incubated on the plate at room temperature for 2 hours. The ELISA plate-coated wells were washed eight times with phosphate-buffered saline (PBS) (pH 7.5 containing 0.05% Tween-20). The wells were then loaded with 100 μL of anti-PMMoV antibody and alkaline phosphatase at 1:1000. The plates were incubated at 37°C for 2 hours, and the wells were washed eight times with PBS solution as mentioned in the previous washing step. The absorbance value of each sample was measured at 405 nm by an ELISA reader (Titertek, Huntsville, AL, USA) thirty minutes after the addition of 100 μL of the substrate (p-nitrophenyl phosphate at 1 mg·mL−1 in 10 % of diethanolamine pH 9.8). A sample was noted as positive if the OD exceeds 3 times the mean of the negative controls (Yoon et al., 2021).
Solvent extraction and GC-MS analysis
To identify the active components in the supernatants of bacterial cultures, a 48-h grown Pseudomonas putida and Bacillus licheniformis broth was centrifugation at 10,000 rpm for 10 min, and the supernatant was collected and mixed with an equal volume of ethyl acetate, as in the ratio of 1:1 (v/v). The mixture was shaken vigorously for 20 min, and by using a separating funnel, the ethyl acetate phase was separated from the aqueous phase. This extraction step is repeated one more time, pooled together with ethyl acetate fractions, and subjected to evaporation at 50°C in a rotary evaporator. The concentrated residue containing the secondary metabolites/chemical compounds was analyzed using gas chromatography-mass spectroscopy (GC-MS). The analysis was run on a GC-MS system (Agilent) and the test was carried out at the Rural Development of Administration. The mass detector was used in split mode and helium gas with a flow rate of 1 mL/min was used as a carrier. The injector was operated at 250°C and the oven temperature for the initial setup was at 60°C for 2 min, scan time 0.2 s, mass range 50 – 650 amu, and ramp 4/min to 250°C for 20 min. Mass spectra were taken at 70 eV, during the running time of 53 min. The constituents were identified after comparing them with available data in the GC-MS library in the literature.
Identified commercial metabolites hypersensitive response on PMMoV in N. glutinosa
The identified three compounds - [cyclo (L-Prol-L-Val), cyclo (Pro-Leu), cyclo (Phe-Pro)] were procured from the Cayman chemical company, USA, and dissolved in the appropriate solvents then diluted to our desired concentrations (10 ppm, 50 ppm, and 100 ppm) with neutral pH. Foliar spraying of all three materials at three different concentrations on N. glutinosa for three days was then followed by PMMoV infection and incubated for 3–5 days under controlled conditions. 1 mM Salicylic acid (SA) acts as a positive control in the experiment.
Local lesion count assay
Infectious PMMoV was quantified by a local lesion count assay in the treated N. glutinosa plants. At the end of the incubation, local lesions on each leaf were counted, and the average lesion count prepared from a single sample was considered as the infectious PMMoV concentration for that sample. The number of local lesions was compared with the control local lesions.
Results and Discussion
Inhibition of PMMoV in chili pepper
Chili pepper plants treated with culture supernatants of Pseudomonas putida and Bacillus licheniformis by foliar spray showed lesser symptoms of PMMoV compared to the non-treated plants after 2 weeks of inoculation. Moreover, the reduction in the severity of PMMoV in chili plants by foliar spray treatment was higher than that of control-treated samples, DAS-ELISA test confirmed that PMMoV titer as an indicator for PMMoV accumulation was markedly reduced by 43–47% in chili pepper plants in both treatments with two culture supernatants (PP, BL of T1 & T2) in comparison to untreated plants after 2 weeks of virus inoculation. The results were expressed in the graphical representation form in Fig. 1. PMMoV accumulation was significantly lower in chili pepper plants with foliar spraying of two bacterial culture supernatants relative to plants treated with distilled water as a control. There are no shreds of pieces of evidence on the inhibition of plant viruses with the materials/mVOCs (Thomas et al., 2020; Tilocca et al., 2020; whereas indirectly inhibit the transmission of viruses from seeds to the plants by numerous compounds/methods. Seed treatment of Acinetobacter strain KNF2022T from tobacco plant roots showed inhibitory effects on the tobacco mosaic virus (TMV) (Lee et al., 2009). A recent report of a soil-inhabiting bacterium, Pseudomonas oleovorans strain KBPF-004 culture supernatants showed antiviral activity in seed transmission of two viruses - PMMoV and cucumber green mottle mosaic virus (CGMMV) (Kim et al., 2017). Recent reports evidenced that, the application of modified whey protein-quercetin bioconjugates suppress the PMMoV infection in chili pepper (Elsharkawy et al., 2022).

Inhibitory activity of bacterial supernatants on PMMoV in chili pepper plants. PP T-1, Treatment of chili pepper plants with supernatants of P. putida 24-hours before PMMoV infection; PP T-2, Treatment of chili pepper plants with supernatants of P. putida 24-hours before and after PMMoV infection; BL T-1, Treatment of chili pepper plants with supernatants of B. licheniformis 24-hours before PMMoV infection; BL T-2, Treatment of chili pepper plants with supernatants of B. licheniformis 24-hours before and after PMMoV infection; PC-Positive control; NC-Negative control.
Identification of metabolites
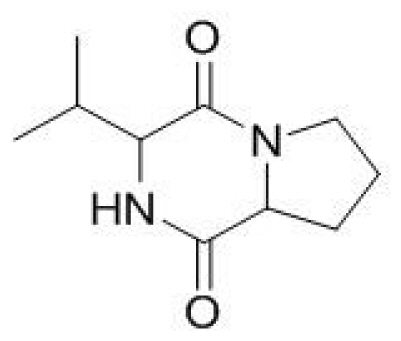
The identification of active components of P. putida and B. licheniformis supernatants after ethyl acetate extract was done by GC-MS analysis. The GC-MS chromatogram for the supernatants of P. putida and B. licheniformis was shown in supplementary Fig. A. The two bacterial supernatants chromatograms illustrated the presence of three metabolites with the different retention times and molecular weights for each metabolite were presented in Table 1. The metabolites were identified with the help of the National Institute of Standards and Technology (NIST) database library, the compounds were cyclo (L-Pro-L-Val), cyclo (L-Leu-L-Pro), and cyclo (Phe-Pro) with the retention time of 41.811, 44.622 and 56.258 respectively. The compound’s mass spectrum structures were presented in Fig. 2. with the values of m/z-196.0, 210.27, and 244.29 in respect of cyclo (L-Pro-L-Val), cyclo (L-Leu-L-Pro), and cyclo (Phe-Pro) (Fig. 2).

GC-MS identification of metabolites in the supernatants of 48-hour grown P. putida and B. licheniformis after ethyl acetate extraction
The three commercial compounds - cyclo (L-Pro-L-Val), cyclo (L-Leu-L-Pro), and cyclo (Phe-Pro) were foliar sprayed on N. glutinosa at three different concentrations inhibit the PMMoV propagation significantly. The hypersensitive response was scored and presented in the graphical form in Fig. 3. The compound - cyclo (L-Pro-L-Val) showed inhibition on PMMoV at the percentage of 52.32, 56.31, and 55.37 in respect to the concentrations of 10 ppm, 50 ppm, and 100 ppm (Fig. 3a). Cyclo (L-Leu-L-Pro) compound also had a similar response to the PMMoV inhibition with 45.66, 63.74, and 65.22 to the concentrations of 10, 50, and 100 ppm (Fig. 3b). In the same way, cyclo (Phe-Pro) hypersensitive response was 57.98, 62.32, and 60.87 to the concentrations of 10, 50, and 100 ppm respectively (Fig. 3c). Overall the three compounds at the concentrations of 10, 50, and 100 ppm inhibited PMMoV in N. glutinosa up to 45–65% compared to the untreated positive control plants. Narrow down to a state that the lowest concentrations of the three compounds 10 ppm and 50 ppm significantly marked the inhibition of PMMoV propagation in N. glutinosa.
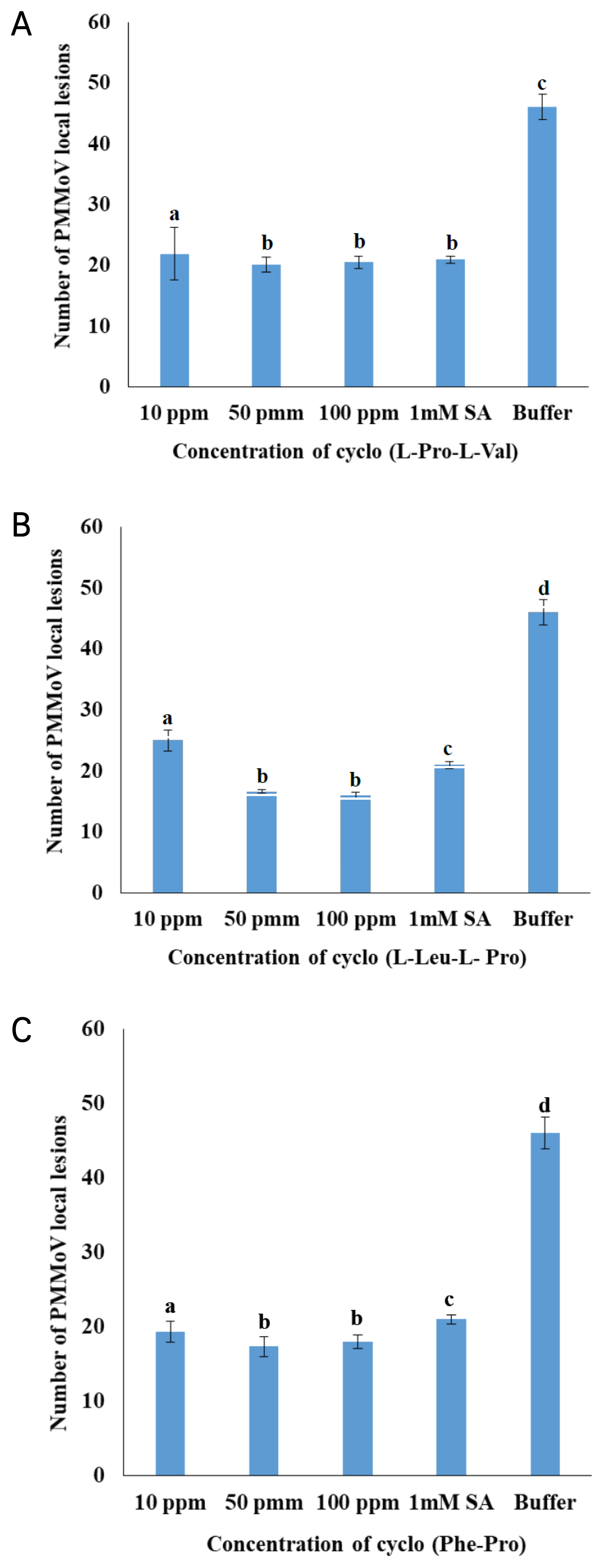
Effect of compounds on PMMoV hypersensitive response in N. glutinosa. (A) cyclo (L-Pro-L-Val), (B) cyclo (L-Leu-L-Pro), and (C) cyclo (Phe-Pro) compounds.
There are surplus number has been reported to control bacterial and fungal diseases by microorganisms or their metabolites/volatiles (Garbeva and Weisskopf, 2020; Li et al., 2022), whilst snippets of information were reported on plant viruses with microbial volatiles. Bacillus species produce a wide structural variability of secondary metabolites that exhibit strong antibacterial and antifungal activities (Stein, 2005; Sansinenea and Ortiz, 2011). Moreover, it represents a new and rich source of secondary metabolites that need to be discovered. Cyclic dipeptides (CDPs) are one of the simplest compounds that are derived from bacteria to humans (Prasad, 1995; Bellezza et al., 2014). Many studies have shown that CDPs possess a variety of biological properties such as antibacterial (Nishanth Kumar et al., 2012), antifungal (Strom et al., 2002), anticancer (Nishanth et al., 2014), neuroprotective (Bellezza et al., 2014), blood-brain barrier transporter (Teixido et al., 2007), anticoagulation (Newman et al., 2003), anti-inflammatory (Minelli et al., 2012), plant growth regulation activity (Ortiz-Castro et al., 2011), triggering plant immunity (Noh et al., 2017), and bio-control activity (Castaldi et al., 2022). The GC-MS chromatogram results revealed that cyclo (L-Prolyl-LValine), cyclo (Pro-Leu), and cyclo (Phe-Pro) were found in Pseudomonas putida and Bacillus licheniformis ethyl acetate extract. In agreement with this result, Bacillus licheniformis strain POT1 ethyl acetate extract GC-MS chromatogram identified the five different chemical compounds, of the five two were the cyclo (L-Pro-L-Val) and cyclo (Phe-Pro). Foliar application of B. licheniformis strain POT1, reduced the alfalfa mosaic virus (AMV) (86.79%) in potato plants and induced systemic resistance in potato plants to AMV (Abdelkhalek et al., 2020). The present study’s experimental results are in agreement with the results of some of the previous reports that indirectly inhibit the virus propagation. Recent evidence unveils that, the endophytic bacteria - Bacillus velezensis Ea73 from Ageratina adenophora, produced two antibacterial peptides - cyclo (L-Pro-L-Val), and cyclo (L-Lue-L-Pro) showed antibacterial activity against S. aureus and E. coli (Ren et al., 2022). A cyclic pentapeptide - malformin A1 from Aspergillus tubingensis FJBJ11 showed potent inhibitory action against the tobacco mosaic virus (TMV) (Tan et al., 2015). Metabolites of Phomopsis sp. FJBR-11, cytosporone U exhibited potent inhibitory against TMV (Tan et al., 2017). The combined action of two cyclic dipeptides - cyclo (L-Leu-L-Pro), and cyclo (L-Phe-L-Pro) could effectively inhibit the growth of vancomycin-resistant enterococci at a MIC value of 0.25–1 mg·L−1 and on pathogenic bacteria - E. coli, S. aureus, and C. albicans at MIC value of 0.25–0.5 mg·L−1 (Rhee, 2004). The two bacterial species - Pseudomonas putida and Bacillus licheniformis culture supernatant could be useful as a preventive agent against PMMoV infection in chili pepper. These results further boost to dig deeper into the bacterial supernatants/secondary metabolites of plant diseases caused by various pathogenic microorganisms. However, further examinations are needed the cross-check this culture supernatants with other plant viruses like TSWV, CMV, and others. However, for the two potential bacterial culture supernatant applications, further examination is needed for the field-level application.
Conclusion
The culture supernatants of Pseudomonas putida and Bacillus licheniformis inhibited the PMMoV propagation in chili pepper in up to 47% of the control plants. Ethyl acetate extraction of culture supernatants by GC-MS analysis identified - cyclo (L-Prolyl-L-Valine), cyclo (Pro-Leu), and cyclo (Phe-Pro) metabolites. These three commercial compounds also relatively showed a hypersensitive of 45–65% to PMMoV in N. glutinosa. These findings reveal that bacterial culture supernatants are useful in controlling PMMoV and minimizing the usage of conventional agrochemicals, which are sinking to the soil and water bodies from the point of environmental safety.