Pilot Study on the Physio-psychological Effects of Botanical Gardens on the Prefrontal Cortex Activity in an Adult Male Group
Article information
Abstract
Background and objective
With modern lifestyles and the increasing severity of stress-related diseases, the availability and accessibility of the natural environment are critical. Botanical gardens in an urban area can provide opportunities for city dwellers to experience nature and enjoy stress-reducing activities. This study focused on the health-related effects of botanical gardens by investigating prefrontal cortical activity and changes in psychological states.
Methods
The experiment was conducted in a national botanical garden located in the city of Sejong, and in an urban area of a contrasting city. Nine healthy adult males participated in the field experiment. Subjects were instructed to 'see and feel' the landscape in a sitting position for 10 minutes, both in the botanical garden and in the city center. The health effects of botanical gardens were verified using Near-Infrared Spectroscopy (NIRS), including changes in cerebral blood flow, and Profile of Mood State (POMS) and Perceived Restoration Scale (PRS).
Results
Our findings showed that the oxyhemoglobin concentrations in the left prefrontal cortex were considerably lower in the botanical garden (−0.057 ± 0.003 μM) than in the city center (0.162 ± 0.002 μM, p < .001). Significantly positive psychological responses to the garden environment were found in the analysis of POMS and PRS, compared to the urban setting. These findings may indicate that a botanical garden can reduce negative psychological symptoms and physiological stress levels in adult males.
Conclusion
Our study proves that botanical gardens have the environmental characteristics of restorative and therapeutic spaces. The findings indicated that urban gardens could be considered as health-improving environments by reducing the physio-psychological stress levels of urban dwellers.
Introduction
In enhancing biodiversity and achieving sustainable development, along with addressing international issues such as climate change response, the role of arboretums and botanical gardens (hereinafter referred to as botanical gardens) is becoming increasingly important. Today's botanical gardens has evolved into a place that performs various social roles that go beyond its past functions as a place for the conservation of plant species, education, and research. Recently, as the public's interest in the environment and their demand for recreational activities related to health promotion has increased, there has been a rising number of people visiting domestic botanical gardens, and the quantitative growth of botanical gardens at the same time (Lee, 2013; Kim and Che, 2018).
This phenomenon allowed botanical gardens, which had been developing mainly on the outskirts of the city, to gradually move into the city center and become located close to the living zone of city dwellers. As an urban green space that can be easily accessed at any time, they have become spaces for relaxation and healing, playing an important role in improving quality of life for city dwellers (Park et al., 2014). In addition, recently, botanical gardens have been created in the form of large greenhouses that can provide an experience environment with the same conditions all year, without being affected by the seasons and climate, expanding the form of use and viewing (Choi et al., 2009). Such an botanical gardens, which is closely located in the city, has the advantage of utilization as a space for nature experience, which can be involved in stress relief and health promotion. In fact, a study on the correlation between health and urban green space reported that walkable green space can extend the lifespan of urban senior citizens (Takano et al., 2002); while Shaw (2015) found that the mental and physical health of visitors improved after activities in botanical gardens.
The artificial environment of modern cities is recognized as an environment that causes risks to human health, with the emergence of 'closed building syndrome' and a rapid increase in diseases caused by social stress, along with various environmental problems related to damage to nature (Lee et al., 2000). Kim et al. (2011) reported that exposure to urban environments increased the level of psychological stress measured by amygdala activity. It was found that cortisol, a stress-related hormone, was secreted more often after activities in cities than in natural areas (Lee et al., 2009). Negative mood states including anxiety, depression, and fatigue also tended to increase after exposure to artificial urban environments (Kessler, 1997; Griffiths et al., 2000; Young et al., 2005). As such, the urban environment is closely related to stress, and thus mental health problems have become one of the important issues for city dwellers (Vlachokostas et al., 2014; Gruebner et al., 2017).
According to researchers in the medical science field, the brain region most closely related to stress is the frontal lobe (FL), which is responsible for human cognitive functions and decision-making; the prefrontal cortex (PFC), located in the front part of the FL, is an important area that has a close correlation with human mental health (Thayer and Brosschot, 2006; Lee et al., 2007; Youn and Yi, 2011; Yang et al., 2019). In this regard, previous studies analyzing the correlation between the PFC and the natural environment secured evidence that blood flow in the FL area decreased after exposure to natural environments such as forests or gardens. These findings have thus demonstrated that the natural environment helps to reduce blood flow in the FL, thereby reducing mental stress (Lee et al., 2015; Song et al., 2018; Song et al., 2020; Ochiai et al., 2020).
However, little research has been done about the health-related value of the botanical garden, a space that provides various benefits to humans, using scientific evidence. Most of the studies conducted on botanical gardens have tended to focus on visitor perception surveys using questionnaires (Hyun et al., 2012; Williams et al., 2015). As a green and cultural space in the city center, botanical gardens have various health effects, providing experiences of various plants in a pleasant environment throughout the year. Verifying these advantages with more quantitative results seems to be very important in determining the future direction of urban botanical gardens. In addition, examining the health effects of botanical gardens related to stress, a major problem in the urban environment, is expected to be one of the major ways to help improve the quality of life and the health of urban residents.
Therefore, this study aimed to investigate the beneficial effects of botanical gardens on stress reduction by examining the FL activity, and to find evidence for health benefits. Based on this, it was sought to derive the healing effects of botanical gardens as a medium for improving health in modern life as a quantitative result. Furthermore, through this study, it is expected that the future direction related to the health function of botanical gardens can be suggested and their development as healing space promoted to improve the health of city residents.
Research Methods
Participants and study sites
In this study, nine Korean adult males (29.6 ± 3.6; mean age ± standard deviation) participated as subjects. Considering that this was a pilot study to investigate the response of the FL in botanical gardens, it was necessary to be able to review the relevance to previous studies and select the appropriate subject group. In this regard, all subjects recruited were healthy males without a history of cardiovascular or psychiatric diseases, excluding variables of physiological response according to gender. The subjects agreed to participate the experiment after being informed of the details of the purpose and content of the study in advance. To control the effect of caffeine, they all did not consume caffeine from the day before the experiment to the day of the experiment. This study was conducted according to the guidelines of the Declaration of Helsinki after being reviewed by the Public Institutional Review Board (P01-202009-12-002).
To verify the health promotion effect of botanical gardens, a field experiment was conducted to compare the psychological and physiological responses in two environments, a botanical gardens and an urbanized space. For the purpose of verifying the health effect of botanical gardens located in the city center, a place with a certain area and universal characteristics as a garden was selected as the study site, including vegetation, water landscape, stones, and flowering plants; an botanical gardens with the shape of a large dome that maintains the same conditions throughout the year was selected to eliminate the environmental changes according to the season(here in after garden). As a universal urban space for the control experiment, a downtown area in Sejong (Naseong-dong) with both residential and commercial functions was selected (Fig. 1; here in after city). It is located about 10 minutes away by car from the selected botanical gardens, the Sejong National Arboretum. The field study was conducted on a fall day suitable for activities, with the temperature ranging from 14–19°C on the day of the experiment.
Experimental protocol and measurements
After being informed of the experiment in the waiting room located in the research building of the Sejong National Arboretum, all subjects moved to the botanical gardens and the downtown one by one. They were requested to wear a masking hat to limit visual stimuli during their trip to the study sites. They were also arranged to arrive at both the experimental and control sites after a 10-minute drive by vehicle in order to make the trip time the same, with the control site located 4.8 km from the waiting room. The experiment was conducted with the subjects seated in a wheelchair to exclude changes in mental and physical responses caused by physical movement; while being seated in a wheelchair, the subjects moved with the support of the researchers. The study protocol is shown in Fig. 2. To offset order effects, participants were randomly assigned to either the experimental site or the control site to take a rest. After arriving at the study sites, the subjects were asked to enjoy the environment while looking at the landscape for 10 minutes while sitting in a wheelchair after a device for measuring the FL response was attached to them (hereinafter referred to as "rest"). The subject's FL responses were continuously measured during the experiment. After a 10-minute rest, a survey of all participants about their psychological state at each site was conducted using a self-reporting questionnaire. After all the experimental procedures at the first site where they arrived were completed, they were moved to the second site via vehicle, where they went through the same experimental procedure as at the first site.
Near-infrared spectroscopy (NIRS) was used to examine changes in cerebral blood flow (CBF) during their rest in the environment of each study site. NIRS is a non-invasive index that measures the concentration of oxyhemoglobin (Oxy-Hb) in the PFC by attaching a device to the forehead of participants. It is also a tool that irradiates near-infrared light to a measurement target and calculates blood flow based on its absorbance. (Holtzer et al., 2011). In this study, an NIRS consisting of a total of 15 channels (NIRSIT LITE; OBELAB, Seoul, Korea) was used.
In addition, the Profile of Mood States (POMS) was used to measure the mood state of participants (Yeun and Shin-Park, 2006). This tool consists of 30 items on a 5-point Likert scale with a total of 6 sub-domains, including tension-anxiety (T-A), anger-hostility (A-H), depression (D), vigor (V), fatigue (F) and confusion (C). The higher the total mood disturbance (TMD) score, the lower the participant's mood is interpreted (Kim et al., 2021).
The Perceived Restorativeness Scale (PRS) was used as a tool to measure the degree of psychological restoration through contact with a given environment. In this study, the Korean version of the PRS consisting of a total of 16 items with four sub-domains including "Being away," "Fascination," "Coherence" and "Compatibility" was used (Lee and Hyun, 2003). Overall satisfaction was surveyed on a 7-point Likert scale to examine the relationship between psychological restoration and satisfaction (Loo et al., 1999; Kim, 2021). The results of reliability assessment (Cronbach's α) for POMS and PRS were found to be 0.835 and 0.962, respectively.
Statistical analysis
To find a significant difference, physiological and psychological data obtained from all subjects at the botanical gardens and the control site were compared. Statistical tests of all collected data were statistically processed using SPSS Statistics 21.0 (IBM, USA). For NIRS consisting of a total of 15 channels, an analysis was conducted based on channels over the whole PFC and the left and right hemispheres, referring to previous studies (Sackeim et al., 1990; Mayberg et al., 1994; Bench et al., 1995; George et al., 1999; Post et al., 1999). Over the whole PFC, all 13 channels (CH2–CH14) were analyzed, except for two error channels (CH1, CH15). Over the left and right hemispheres, a total of 1,229,760 data of 6 channels over the left hemisphere (CH2–CH7) and 6 over the right hemisphere (CH9–14) were analyzed, excluding a center channel (CH8) and two error channels (CH1, CH15). For the analysis of means (ANOM), the mean of Oxy-Hb values during 10 minutes of rest in a given environment was calculated. For time series analysis, the mean of data measured for one minute immediately before rest was set as a baseline, and data during rest was split into and averaged by 60 second intervals. For an analysis with high reliability, the statistical method of the data was applied by referring to previous studies. Studies using the same index (NIRS) as that used in this study were reviewed. Most of the previous studies were found to use parametric tests when analyzing blood flow data in the PFC, including a study comparing the psychological effects of seeing forests and urban landscapes for seven adults, and a study of such effects of experiencing forest and a control site for 15 adults. Based on this, the paired t-test was applied as a statistical method for analyzing blood flow changes in the PFC (Horiuchi et al., 2014; Joung et al., 2015; Lee, 2017; Song et al., 2018). With reference to studies by Goto et al. (2013) and Kim et al. (2021), which assessed emotional responses in natural and control environments, psychological data was analyzed using the Wilcoxon signed-rank test by applying non-normal distribution according to the nature of the data. Data of all results were expressed as mean ± standard error, and the statistical significance level was set to p < .05.
Results
Change in frontal cortical blood flow
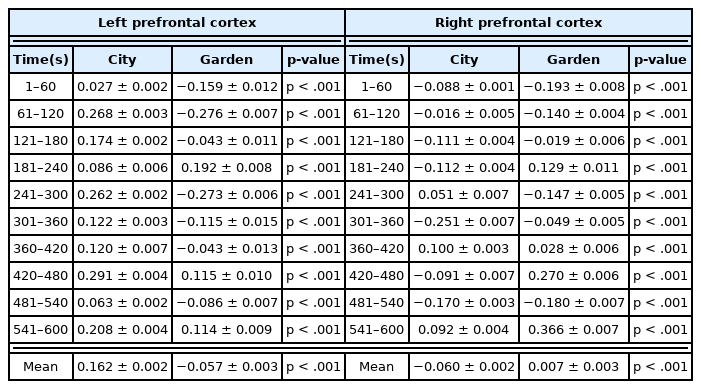
In an analysis of total blood flow changes in the PFC during resting in two different environments, in all participants, measured Oxy-Hb concentration in the PFC was twice as low in the garden environment of the botanic garden compared to the urban environment (City: 0.041 ± 0.002 μM, Garden : −0.032 ± 0.003 μM, p < .001). This means that rest in the garden of the botanic garden helped stabilize CBF compared to the control environment, suggesting that the garden is an effective environment for stress relief and physiological stability (Fig. 3a). In this study, to examine the response of the FL more closely, an analysis was carried out by dividing it into left and right hemispheres. The results of this analysis also showed a significant difference in the change of Oxy-Hb concentration in the left and right PFCs. When comparing the means of 10-minute data, Oxy-Hb concentration in the left PFC was significantly decreased in the experimental site (Garden) compared to in the control site (City) (City: 0.162 ± 0.002 μM, Garden : −0.057 ± 0.003 μM, p < 0.001). In contrast, a significantly higher blood flow response in the right PFC was observed in the garden (City: −0.060 ± 0.002μM, Garden: 0.007 ± 0.003 μM, p < .001, Fig. 3b).
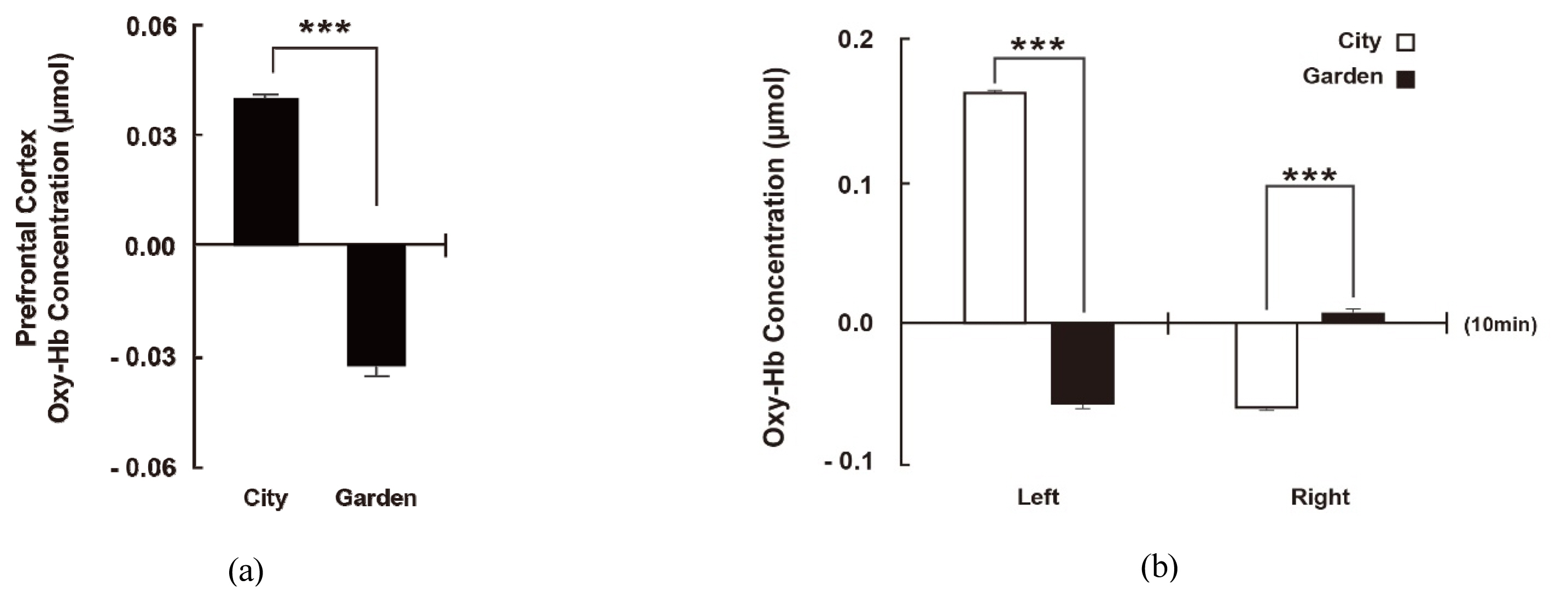
Overall mean oxy-Hb concentrations. Data are expressed as means ± standard error; n = 9, *** p < .001, as determined by the paired t-test (one-sided); the Holm correction was applied.
The data on the change in Oxy-Hb concentration in the left PFC measured during 10 minutes of rest in the city or garden was split into and averaged by 60 second intervals, which are shown in Table 1. Immediately after the start of rest (1–60 seconds), Oxy-Hb concentration in the left PFC was lower in the experimental site (Garden) than in the control site (City) (City: 0.027 ± 0.002 μM, Garden: −0.159 ± 0.012 μM, p < .001). Thereafter, low changes in blood flow in the left PFC were observed for all sections, with the exception of the 180–240 second section (City: 0.086 ± 0.007 μM, Garden : 0.192 ± 0.008 μM, p < .001). This result is the same as that of comparing the means of the 10-minute data analyzed above. On the other hand, in the right PFC, immediately after the start of rest, the Oxy-Hb concentration in the control site (City) was increased compared to the experimental site (Garden). However, over time, the blood flow tended to increase in the experimental site (Garden) compared to the control site (City) (Table 1). When the last section of 541–600 seconds was compared with the first section, the blood flow increased to 0.092 ± 0.004 μM in the city compared to the baseline, but in the garden, the blood flow increased to 0.366 ± 0.007 μM, showing a much larger increase.
Psychological measurement
By analyzing the POMS data, the participants were found to show a more positive mood state in the garden than in the control site (City: 11.89 ± 6.23, Garden: −4.78 ± 2.34, p < .05). Of the sub-scales of POMS, the score of negative mood states was significantly lower in the garden than in the city: T-A(City: 4.89 ± 1.40, Garden: 0.67 ± 0.44, p < .05), A-H(City: 2.67 ± 1.13, Garden: 0.22 ± 0.15, p < .05), and F(City: 3.67 ± 1.21, Garden: 2.33 ± 1.01, p < .05). In addition, the score for vigor (V), a positive mood state, was significantly increased during rest in the experimental site compared to the control site (City: 6.33 ± 1.88, Garden: 11.67 ± 1.47, p < .05). The other sub-domains, depression (D) and confusion (C), showed lower scores in the garden than in the city, but the difference was not statistically significant (Fig. 4).

POMS scores in the two environments (city and garden). The data are expressed as means ± standard error; n = 9, * p < .05, as determined by the Wilcoxon signed-rank test.
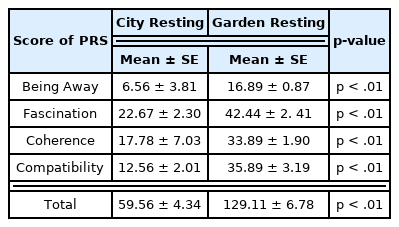
Based on the scores of the PRS surveyed after rest in the control and experimental sites (City and Garden, respectively), the participants gave a higher score for psychological restoration after rest in the garden (City: 59.56 ± 4.34, Garden: 129.11 ± 6.78, p < .01, Fig. 5). In all four subscales of the PRS (Being away, Fascination, Coherence, Compatibility), it was found that the participants tended to give higher scores for rest in the garden than that in the city (Table 2). This suggests that the garden is a relatively better environment for psychological restoration compared to the city.

PRS scores in the two environments. The data are expressed as means ± standard error; n = 9, ** p < .01, as determined by the Wilcoxon signed-rank test.
Discussion
This pilot study was conducted to obtain evidence to support the benefits of botanical gardens in terms of mental health. To this end, we sought to increase the reliability of the results by using both biomarkers related to the central nervous system and self-reported psychological indexes.
Previous studies analyzing CBF responses in natural environments including forests have reported that experience in natural environments can promote biological relaxation by stabilizing FL activity. Park et al. (2007) reported that walking in a forest for 15 minutes decreased the total hemoglobin concentration in the left PFC compared to walking in the city center. Joung et al. (2015) proved that seeing a forest environment has the effect of lowering the total hemoglobin and Oxy-Hb concentrations in the PFC compared to seeing the city. In addition, Lee (2017), who analyzed CBF responses in gardens, found the visual landscape of gardens tended to lower the average concentration of Oxy-Hb compared to that of the urban landscape, establishing the tendency of changes in CBF in the natural environment.
At the same time, these researchers contended that experiences in the natural environment have the effects of improving the psychological state in a positive direction, and proved that there is a close relationship between the physiological and the psychological-emotional state. By comparing the effects of the visual environment of forests and cities, Lee et al. (2009) demonstrated that the higher the PRS score, the higher the electroencephalogram (EEG) increased, which helped decrease the CBF and alleviate negative emotions. The findings were also found in a study by Chang et al. in 2008: the higher the PRS score, the higher the electroencephalogram (EEG) and electromyogram (EMG) tended to increase and the blood pressure to decrease. Joung et al. (2015) reported that seeing forest landscapes not only decreased the blood flow in the PFC, but also the scores of negative sub-scales of the POMS decreased and those of positive sub-scales improved. In addition to these findings, most studies have suggested that experiences in the natural environment can give physiological and psychological relaxation effects through emotional relief, and reduction of CBF (Song et al., 2018; Song et al., 2020; Kang et al., 2020; Ochiai et al., 2020). This study also showed the same results, proving that the botanical garden environment can help improve the emotional state along with the decrease in CBF.
In this study, we quantitatively analyzed the health effects of an botanical garden by referring to the results of previous studies, deriving significant findings that were in line with those. Based on the results of analysis of CBF response using NIRS, rest in the botanical garden was found to be effective in relieving mental and biological stress by significantly reducing the Oxy-Hb concentration in the whole PFC and left PFC, compared to rest in the city. These results can be said to be consistent with a previous research finding in cognitive neuroscience, which is that for healthy general adults, CBF decreases in a state in which stress is relieved (Sackeim et al., 1990; Mayberg et al., 1994; Bench et al., 1995; George et al., 1999; Post et al., 1999). In addition, regarding psychological responses, negative mood states including tension and anxiety, anger and hostility, and fatigue after rest in the botanical garden were alleviated, and at the same time, positive mood states including vigor were improved compared to urban environments. As the subjects gave high scores for rest in the botanical garden as a restorative environment, it was also found that the natural environment located close to cities can serve as a restorative tool for urbanites experiencing high levels of stress.
Comparing the results of previous studies with those of this study, it appears that the mental and physical responses in the garden are consistent with those in the forest, a relatively large-scale environment. This indicates that activities in an botanical garden may have similar health effects to those in a forest. This finding may serve as a scientific basis for proving that botanical garden environments are an excellent restorative environment that promote the stabilization of blood flow changes in the PFC, and psychological and emotional stability. It is expected to be important reference material when planning the use of botanical gardens as a healing space in the future.
On the other hand, the findings of this study related to the right PFC differed from those of previous studies. It is known that the blood flow in the right PFC of normal people often increases in response to environmental stressors (Tennant, 2002; Kim, 2006; Lee, 2008). Some previous studies have reported that blood flow in the right PFC decreases when exposed to natural environments (Song et al., 2018; Song et al., 2020). The reason for this difference from the results of previous studies is related to the response characteristics of the right PFC. The right PFC is known as the part of the brain most sensitive to stress, but at the same time, it has a wide range of variations in its blood flow responses, which has led to various research findings (George et al., 1999; Post et al., 1999). For this reason, it seems that there is a limit to our ability to draw generalized conclusions about the right PFC. Nevertheless, this study has significance: regarding the blood flow responses of the whole and left PFC, results consistent with previous studies based on various natural environments were derived, and the health function of botanical gardens was quantitatively verified.
Although botanical gardens today play a social role as a place of healing for health promotion as well as leisure and education, research on health effects and scientific evidence are still insufficient. Since this study is a pilot study focusing on the stress response of the cerebral frontal lobe, an approach that extensively verifies the health effects using various indexes of mental and physical responses is required in the future. In addition, it is necessary to systematically determine the health function of botanical gardens through additional research by increasing the subject group and size and expanding the study sites.
Conclusion
The results of this study found that a garden in an botanical garden can reduce physiological stress and promote psychological stability, and provided a scientific basis for the response of the cerebral frontal lobe. It was suggested that botanical gardens can be used as a healing space for adult males, through their effect of significantly lowering the concentration of Oxy-Hb in the whole and left PFCs and improving the psychological state compared to urban environments. The POMS results proved that botanical gardens help significantly improve the negative mood state and have a positive psychological state. All of the sub-domains of the PRS were assessed with high scores, proving that botanical gardens are an excellent restorative environment compared to general urban environments. These results prove that since an botanical garden environment stabilizes blood flow responses in the FL and induces a psychological stabilization effect, it is an environment with healing factors that induce mental and physical stability by lowering negative emotions, including depression and stress. To overcome the limitations of this as a pilot study, research to verify changes in cerebral function for several user groups should be conducted. Furthermore, a systematic and careful approach to examining the health benefits of various botanical gardens will be required.