Effects of Nutrient Solution Application Methods and Rhizospheric Ventilation on Vegetative Growth of Young Moth Orchids without a Potting Medium in a Closed-Type Plant Factory
Article information
Abstract
Background and objective
Moth orchids in the vegetative stage are suitable for a multi-layer growing environment in a closed-type plant factory which can be a good alternative that can reduce production costs by reducing cultivation time and energy cost per plant. This study was conducted to find out the optimal rhizospheric environment for different irrigation methods without a potting medium and rhizospheric ventilation for the vegetative growth of young Phalaenopsis hybrid ‘Blanc Rouge’ (P. KV600 × P. Kang 1) and Phalaenopsis Queen Beer ‘Mantefon’ in a closed-type plant factory system.
Methods
The one-month-old clonal micropropagules with bare roots rapped with a sponges were fixed on the holes of styrofoam plates above growth beds, and were watered using the ebb-and-flow (EBB) and aeroponic (AER) methods with Ichihashi solution (0.5 strength) once a day at 06:00 (P) or 18:00 (S), and both (PS). Rhizospheric ventilation (V) was also applied to change the temperature, relative humidity, and CO2 concentration of the beds. Plants potted into sphagnum moss and watered once a week were used as the control group.
Results
After 12 months of treatment, the growth characteristics of the EBB groups were the best among the treatment groups without a medium, but no effect of irrigation timing was observed. V reduced the temperature, relative humidity and CO2 concentration of the beds. Whereas, EBB+V (ebb-and-flow with ventilation) improved plant growth and reduced the occurrence of disorders and withering. Especially, EBB+V showed a similar performance to the control group.
Conclusion
The results indicated that the optimal irrigation method without a potting medium for producing middle-aged potted moth orchids was the EBB system with forced rhizospheric ventilation. Therefore, further studies on the optimal ventilation method and moisture control of the crown need to be carried out to develop the irrigation system without a potting medium for vertical farming in closed-type plant factories.
Introduction
Moth orchids as a species of orchids are epiphytic plants native to tropical regions. Since it takes a long time to bloom, tissue culture technology can be used to shorten their growth period, but it still takes 18 months from clonal micro-propagules to harvest them (Lee et al., 2010a; Rural Development Administration[RDA], 2018). The effective temperature for the vegetative growth of moth orchids is over 28°C, and they must be treated at 23°C or lower to induce blooming (Lee et al., 2015). In addition, as most of their clonal micro-propagules are imported from other countries such as Taiwan, and in-vitro propagules are used, the share of seedling, unpaid labor and light, heat and power costs accounts for 19%, 22% and 19% of the cost of production respectively.
Due to their characteristics as an epiphytic plant, moth orchids are cultured mostly in sphagnum moss or bark media when pots are used (Hwang and Jeong, 2007; RDA, 2018). In general, clonal micro-propagules are planted in plug trays or small-sized plastic pots with sphagnum moss from the stage of ex-vitro acclimatization, and are later transplanted into larger-sized pots or media (Lee et al., 2010a). In the process of planting, a significant amount of labor is required, and the cost of purchasing sphagnum moss that is entirely imported accounts for a large share of the total cost of production (Wang and Gregg, 1994). In an environment where moth orchids can be grown without a medium, their characteristics as an epiphytic plant need to be utilized. In this case, the cost of distribution can be reduced, and they can be conveniently planted in various types of media in the stage of distribution or consumption, which is worth consideration. In particular, this method seems to be highly applicable to mini- or small-flowered moth orchids with a short growth period.
Compared to other flowers, the market price of moth orchids tends to be constant throughout the year in the domestic market (Ministry of Agriculture, Food and Rural Affairs[MAFRA], 2015). Since their plant length is low until they reach floral differentiation, they are suitable for multi-layer cultivation. As they grow in an shaded or half-shaded environment and need a low amount of light, artificial light sources can be used and it is also easy to control temperature required to induce vegetative growth and blooming within facilities (RDA, 2018). For this reason, growth conditions can be easily controlled with the method compared to greenhouses and it is also advantageous to produce them year-round and utilize spaces effectively, which indicates that multi-layer plant factories are more suitable for moth orchids. Recent abnormal weather events in an external environment such as changes in temperature and the amount of light are also disadvantageous, which has increased the necessity of studying multi-layer closed plant factories where moth orchids can be grown in an isolated environment.
Therefore, it is recommended to introduce plant factory technology in order to reduce the costs of producing moth orchids and shorten their growth period through the minimized movement of workers and precise environmental control. If the system does not require a potting medium, its efficiency will be improved. There have been also attempts to grow plants in the basements of buildings or in small-sized spaces in cities and thus to attract consumers’ interest, indicating that the introduction of closed plant factory systems for flowering plants can be a good alternative in urban agriculture.
There are earlier studies on conditions for the underground part of moth orchids such as the composition and concentration of nutrient solutions in a greenhouse environment (Wang, 1996; Hwang and Jeong, 2006, 2009; Hwang et al., 2004, 2009; Lee et al., 2010b); media (Hwang and Jeong, 2007; Hwang et al., 2004; Kim et al., 2016; Wang, 1995, 1998; Wang and Gregg, 1994); and irrigation methods (Lee et al., 2010a; Lee and Lee, 2004). There were also studies on growing epiphytic plants without a potting media including Dendrobium (Chung et al., 1997) and moth orchids (Lee et al., 2010a). These studies, however, were conducted only in greenhouses, and no study has been conducted on the management of the underground part of moth orchids within a closed plant factory that utilizes artificial light only. This study aimed to find the optimal environment for the underground part of middle-aged moth orchid micro-propagules without a potting media by identifying the effects of the methods and time of supplying a nutrient solution and rhizospheric ventilation on their growth, and thus to provide basic information for the development of closed plant factory systems.
Research Methods
Plant materials
The clonal micropropagules of Phalaenopsis hybrid ‘Blanc Rouge’ (P. KV600 × P. Kang 1) and Phalaenopsis Queen Beer ‘Mantefon’ that were acclimatized ex-vitro were purchased from a moth orchid farm (Sangmiwon, Taean, Korea) and those of a uniform shape were selected as a plant material. They were originally planted in a sphagnum moss medium, and thus sphagnum moss was carefully removed not to damage their roots for this experiment. After that, their roots were wrapped and fixed with a round-shaped sponge (diameter: 6 cm, thickness: 2 cm), and were planted in a styrofoam bed within a round-shaped hole (L60 × W60 × H2 cm).
Closed plant factory system
This experiment was conducted in a container-type plant factory installed within the farm of the College of Life and Applied Science of Yeungnam University (Fig. 1). The inner temperature and relative humidity of the closed plant factory installed within the container (L8.0 × W3.0 × H2.8m) were maintained at 29 ± 1°C and 70 ± 5% respectively, and the warm-white LED (Fig. 1) was used as a source of light under the following lighting conditions: photosynthetic photon flux density (PPFD), 150 μmol·m−2·s− (at the height of leaves); and day length, 12 hours (photoperiod: 06:00–18:00, scotoperiod: 18:00–06:00). The temperature and humidity of the container were controlled using an air-conditioner, heater and humidifier, and the growth environment for the aboveground and underground part of plants was measured and recorded using a CO2 recorder (TR-76Ui, T&D Inc., Japan), data logger (Almemo 2690-8A, Ahlborn, Holzkirchen, Germany), psychrometer (FNAD-46, Ahlborn) and CO2 sensor (FYAD00, Ahlborn). The concentration of CO2 was not separately controlled but was maintained at 400±50 ppm.
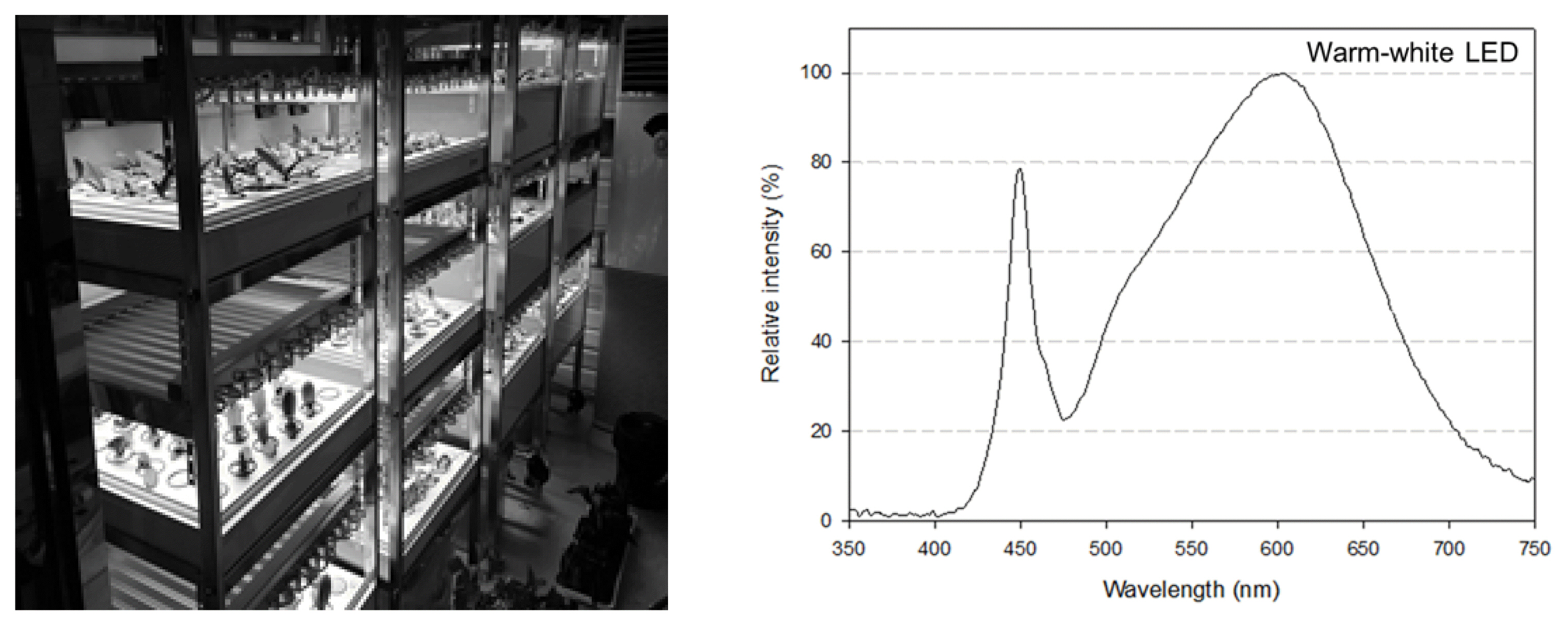
The closed-type plant factory system (left) and light spectrum of warm-white LEDs (right) used in this experiment.
In this study, 6 shelves with 3 layers for each shelve were installed (Fig. 1), and the frequency and time of irrigation was separately controlled for each layer. On each layer 4 cooling fans commonly used for computers were installed to ensure there was no difference in temperature, humidity and CO2 concentration between top and bottom and left and right parts. On the floor of the plant factory, 2 small-sized fans were placed for ventilation for 24 hours.
Irrigation methods and rhizospheric ventilation
In this study, 2 types of irrigation method were selected as follows: the ebb-and-flow (EBB) method using a self-produced PVC bed (L60 × W60 × H6 cm); and the aeroponic (AER) method using a bed (L60 × W60 × H18 cm) of which height w as 3 t imes h igher than t hat of E BB. A s the control group, those planted in a sphagnum moss medium were placed in the same bed of the EBB method. The nutrient solution used in this experiment was the Ichihashi solution diluted 1/2 times (Ichihashi, 1997), and the pH and EC level of the solution was maintained at 6.0±0.1 and 0.8±0.1 dS·m−1 respectively. To secure the consistency of the experiment, the solution was replaced once every week.
Irrigation time and frequency
Irrigation was applied to each method at 3 different times as follows: one time (P) for 90 seconds at 06:00 when the photoperiod began; one time (S) for 90 seconds at 18:00 when the scotoperiod began; and two times (P + S) for 90 seconds at 06:00 and 18:00 respectively (P + S). During the EBB treatment, the nutrient solution was supplied so that the roots were sufficiently immersed, and the spraying direction in the AER was adjusted so that the sprayed nutrient solution sufficiently wet the roots. For the control group, the same nutrient solution used for other treatment groups was injected into its medium once a week. Since roots were rotten when they were watered more frequently in a preliminary experiment, the time and frequency of irrigation above were selected.
Rhizospheric ventilation
Air was injected (10 L·min−1) to one bed for each irrigation method using an air pump (LP-40A, Gosung, Korea), while irrigation one time during the scotoperiod, and the other bed was not injected with air. In the experiment that was conducted without rhizospheric ventilation above, rotten roots were observed in many areas, and this symptom seemed to be related to the gradual increase in the air temperature, CO2 concentration and relative humidity of the rhizosphere after irrigation. For this reason, the rhizosphere was treated with ventilation. The air temperature, CO2 concentration and relative humidity of the rhizosphere were measured from 30 minutes before the 10-minute ventilation (air injection) until 120 minutes after that.
Growth characteristics survey and statistical analysis
On one bed of each treatment group in the two experiments above, 18 plants of ‘Blanc Rouge’ and ‘Mantefon’ respectively, a total of 36 plants, were placed. After 8 weeks of treatment, 5 plants were randomly sampled for each treatment group and the length and width of the upper-most mature leaf, the number of leaves and roots (longer than 0.5 cm) and the length of roots were measured. In addition, the number of plants that showed growth disorders or withered was counted for each treatment group. Disorder on plants was calculated as a percentage by dividing the number of leaves with morphological abnormality, spots, and decay for each individual by the total number of leaves, and the mortality rate was expressed as a percentage by dividing the number of dead individuals by each treatment group by the total number of individuals.
The collected data were statistically analyzed using the SPSS program (IBM SPSS Statistics 23, IBM, USA), and two-way ANOVA was conducted to verify the main effect and interaction effect of each factor (treatment). Duncan’s multiple range test (p < .05) was conducted to analyze the significance of differences between treatment groups.
Results and Discussion
Effects of the methods and time of supplying the nutrient solution
The effect of the methods of supplying the nutrient solution (irrigation) was overall higher in both species cultured without a potting medium than the time of irrigation (Table 1). In particular, Phalaenopsis ‘Blanc Rouge’ showed the higher effect of the method of supplying the nutrient solution, and the number, length and width of leaves in the aboveground part of the plants in the EBB groups were higher than those in the AER groups. The number and length of roots in the underground part were also higher in the EBB groups than the AER groups, and the rate of withering was also lower in the EBB groups (Table 1). Those planted in a sphagnum moss medium (control group) showed a higher or similar level in the growth characteristics or the rate of disorders and decomposition than all the treatment groups without a potting medium.

Effect of irrigation method and timing on growth and morphological characteristics in Phalaenopsis hybrid ‘Blanc Rouge’ (P. KV600 × P. Kang 1) and Phalaenopsis Queen Beer ‘Mantefon’ at 16 weeks after treatment
The effect of the methods of irrigation in the case of Phalaenopsis ‘Mantefon’ was also higher, but the effect of the time of irrigation or that of interactions between the two elements were found to be higher than the case of ‘Blanc Rouge’ (Table 1). In the case of ‘Mantefon,’ the number o f leaves was h igh in the entire EBB groups. In leaf length, the EBB-PS treatment showed higher values than all treatments except the control, and the EBB-P group had longer leaves than the AER-P and AER-S groups. The width of leaves was high in the EBB-P and EBB-S groups. The number of roots was high in the EBB-P, EBB-S, AER-P and AER-S groups, and the length of roots was high in the entire EBB groups and the AER-S group. The fresh and dry weights of shoots and roots showed a similar tendency to the morphological characteristics of the leaves and roots. Overall, plant mass of the control group was the largest, and that of the EBB groups was higher than the AER ones.
In overall, the EBB groups showed a higher growth both in the aboveground and underground part of plants, indicating that both ‘Blanc Rouge’ and ‘Mantefon’ were affected more significantly by the methods of irrigation than the time of irrigation (Table 1). When growing plants, it is essential to meet their water requirement, and CAM (crassulacean acid metabolism) plants such as Kalanchoe show a decrease in photosynthesis when the amount of water absorption is reduced by a lack of hydration (Rabas and Martin, 2003). In this study, the roots of moth orchids, a CAM plant (Martin et al., 2010; Ota et al., 1991), in the AER groups with a low growth were highly unlikely to be fully soaked, and thus it can be inferred that those in the AER groups were not sufficiently watered or did not sufficiently absorb water.
Moth orchids as an epiphytic plant have roots covered with sponge-like tissues called velamen, and these tissues are composed of 2–3 tissue layers and thus can store as much water as possible by minimizing the loss of water (Augustus and Knudson, 1957). These tissues, though the roots of moth orchids are exposed to air, can adequately absorb water and nutrients, and thus the roots of moth orchids require more air (oxygen) than general terrestrial plants. For this reason, it is important to balance the supply of water and oxygen, and this is highly affected by the microclimate surrounding their roots. The microclimate surrounding the rhizosphere of plants was compared depending on the method of irrigation. The concentration of CO2 in the EBB and AER groups was similar, while the temperature in the AER groups and the relative humidity in the EBB groups were higher (Fig. 2). These structural characteristics of the roots of moth orchids and differences in the rhizospheric environment between the methods of irrigation seemed to affect their growth characteristics or the rate of disorders and decomposition. In particular, the high temperature in the AER treatment beds seems to increase the respiration rate, which can be interpreted as a result similar to the report that the plant growht became poorer with increasing the rhizosphere temperature (Lee et al., 2004).

Changes according to time in rhizospheric CO2 concentration (A), temperature (B), and relative humidity (C) by forced ventilation into root zone in moth orchids hydroponically grown in a closed-type plant factory system. Nutrient solution was applied by EBB and aeroponic (AER) systems.
Lee et al. (2010a) varied the interval of irrigation to moth orchid propagules in aeroponics, and reported that when the nutrient solution was intermittently sprayed on them for 12.5 seconds every 20 seconds repeatedly all day, their growth was the best. The study was conducted in a greenhouse with very different conditions from those applied to this study, which seemed to result in differences. In a greenhouse, roots are dried within 30 minutes, but its relative humidity is high and their rhizosphere is closed to some extent. However, some parts of the plants were rotten due to excessive moisture despite 1–2 times of irrigation (spraying) per day, and the sponge fixed around the crown of plants where roots and stems meet seemed to induce excessive moisture.
As a result, it is important to make sure roots are fully soaked when irrigation. It would be better to increase the time of irrigation in aeroponics, but there was no significant increase in growth when irrigation two times (PS) compared to irrigation one time per day (P, S). Therefore, it seems to be better to select a method of irrigation in which the nutrient solution can be absorbed by velamen tissues well. If it is possible to control overhydration in the crown while fully soaking the velamen tissues, the growth of moth orchids can be increased further and the decomposition rate is expected to decrease.
Meanwhile, there was no statistically significant difference between the control group (planted in a sphagnum moss medium using the conventional method) and the groups that were most effective among those without a potting medium such as the EBB-S group of ‘Blanc Rouge’ or the EBB groups of ‘Mantefon’ (Table 1). The cause of the result can be found in the study (Lee et al., 2010a) that reported normal roots grown within a potting medium absorbed more water than aerial roots grown outside the medium (Lee et al., 2010a). That is, most of the roots of the plants in the control group were within a potting medium and thus are normal roots, while the roots of plants grown without a potting medium were partially normal roots (grown prior to this experiment), and the rest roots (grown after this experiment) were aerial roots. Therefore, those in a potting medium with more normal roots seemed to absorb more water.
Environmental changes within a bed depending on irrigation methods and rhizospheric ventilation and growth characteristics of middle-aged propagules
Changes in the CO2 concentration, temperature and relative humidity of the rhizosphere (within a bed) depending on forced ventilation (air injection) are as shown in Fig. 2. The CO2 concentration of plants watered using the two methods before forced ventilation was similar, and the CO2 concentration right before irrigation (1,000–1,300 ppm) was dropped to under 700 ppm after forced ventilation. The concentration was maintained for the next 2 hours. The temperature of air before ventilation was 30°C in the EBB bed, and 31°C in the AER bed, slightly higher than that in the EBB bed. After ventilation, the temperature in the EBB and AER beds decreased by 2.5°C and 2°C respectively and started to gradually increase after that. The relative humidity before ventilation was 98% in the EBB bed, slightly higher than that in the AER bed (95%). After ventilation, the relative humidity decreased by 6~7% into 91% and 89% respectively and started to gradually increase after that.
In a greenhouse, the CO2 concentration can be increased up to 800–1,600 ppm by carbon dioxide fertilization (RDA, 2018), and thus 1,300 ppm does not seem to be excessively high. However, the bed used in this experiment was placed in a closed space and this is likely to reduce the concentration of oxygen. The optimal relative humidity for moth orchids is 70%, and their growth is reduced at a lower relative humidity (Lee et al., 2018; RDA, 2018). Given that, the optimal relative humidity for the roots of moth orchids as an epiphytic plant seems to be about 70%. There has been no report on a higher relative humidity, but 90% of relative humidity at the rhizosphere of plants is likely to be higher than the optimal range. The optimal temperature for moth orchids in the vegetative growth period is 29°C (RDA, 2018), but the temperature at the rhizosphere of plants within the AER bed was slightly higher.
Rhizospheric ventilation created a similar environment both in the EBB and AER groups under different air injection conditions, but the range of the change in the AER groups was relatively small (Fig. 2). This can be attributed to the size of the AER bed, three times bigger than that of the EBB bed, which seemed to make it difficult to replace the entire air by injecting air only one time. In addition, the temperature in the EBB bed of which size was smaller was lower by 1–2°C, which can be also attributed to the d ifference in the s ize of beds. S ince EBB beds are filled with the nutrient solution at a lower temperature than the rhizosphere of plants and the solution is withdrawn from the beds, a large volume of water stays around the rhizosphere of plants in the relatively small-sized beds for a long period of time, which seems to make the temperature slightly lower (RDA, 2018), and humidity slightly higher. The concentration of CO2 was s lightly h igher in the EBB beds of which volume was smaller in this study, which can be attributed to the fact that a similar volume of roots exist in a smaller space, generating more CO2.
The methods of irrigation and rhizospheric ventilation affected the number and size of leaves, the number and length of roots and the rate of disorders and withering (Table 2). ‘Blanc Rouge’ showed a better growth in the EBB groups in overall than the AER groups, and the positive effect of forced ventilation was observed in the EBB groups. The EBB+V (forced ventilation) group showed the best results in the number, length and width of leaves, the length of roots, the fresh and dry weights of shoots and roots, and the rate of disorders and decomposition, similar to the conventional method using a sphagnum moss medium, while the AER+V group showed the worst results (Table 2). ‘Blanc Rouge’ in the EBB groups with forced ventilation (V) did not show a statistically significant improvement in their growth compared to the groups without ventilation (N), but the overall growth indicators tended to increase and the rate of disorders or withering was reduced (Table 2). Whereas, forced ventilation in the AER groups resulted in negative effects such as a decrease in the length of roots and an increase in the rate of disorders. The AER+N group also does not seem to sufficiently access water or absorb water through roots, and the changes in the environment, such as the airflows generated by the forced ventilation (air injection) and during the nutrient solution injection in AER, seems to cause moisture loss in the roots.

Effect of irrigation method and rhizospheric ventilation on growth and morphological characteristics in Phalaenopsis hybrid ‘Blanc Rouge’ (P. KV600 x P. Kang 1) and Phalaenopsis Queen Beer ‘Mantefon’ at 16 weeks after treatment
‘Mantefon’ also showed a better growth in the EBB groups that the AER groups in overall, and the effects of forced ventilation were also similar to ‘Blanc Rouge’ (Table 2). Most of the growth characteristics showed the best results in the EBB+V group similar to the control group, and the worst results in the AER+V group, indicating that forced ventilation can affect aeroponics negatively. The EBB+N and AER+N groups showed similar characteristics, and the EBB+N group showed a significant improvement only in the number of roots and the rate of withering (Table 2). Only when the roots of ‘Mantefon’ propagules were short, some roots of the plants in the EBB groups showed disorders or withering as they were soaked in the nutrient solution.
That is, both ‘Blanc Rouge’ and ‘Mantefon’ in the EBB groups showed a better growth both in the aboveground and underground parts in overall, and rhizospheric ventilation seemed to have a positive effect on the growth of plants and the rate of disorders and decomposition in the EBB groups, but a negative effect in the AER groups. Despite the result that the growth characteristics of the EBB groups were better than other groups without a potting medium, they were not better than those planted in a sphagnum moss medium in the control group. This can be attributed to the characteristics of the method that does not use a potting medium but have more aerial roots (Lee et al., 2010a), or to the velamen tissues of roots that were not sufficiently supplied with moisture or the characteristics of the air environment surrounding the rhizosphere of plants that was closed to some extent.
Conclusions
As a basic study on the production of middle-aged moth orchid propagules without a potting medium in a closed plant factory, the effects of the methods and time of irrigation and forced ventilation in the rhizosphere of young plants were examined in this study. In terms of the methods of irrigation, the EBB method showed better results, but there were no significant difference the times of irrigation. Forced v entilation w as e ffective in t he E BB groups, b ut the AER groups rather showed a slightly poorer growth. As a result, subsurface irrigation without a potting medium did not show better results than the conventional method of planting in a sphagnum moss medium (control group), but the EBB+V groups showed similar characteristics to the control group. Therefore, when utilizing the methods without a potting medium, the EBB irrigation method is recommended, and planting plates need to be structured to ensure air can smoothly flow in and out of beds. This can prevent an increase in humidity and temperature in the rhizosphere of plants, and sufficiently supply oxygen, creating an optimal rhizospheric environment. In addition, as the study on the photosynthesis of moth orchids reported, it can be considered to expose roots to light when using the EBB irrigation system. It will be also necessary to conduct an additional study to identify the optimal subirrigation method and the optimal moisture content within a medium in the case of using sphagnum moss like the conventional method or an alternative medium in a plant factory.
Notes
This research was supported by Export Promotion Technology Development Program (317020-5), Ministry of Agriculture, Food and Rural Affairs (Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry).