Porphyrin Biosynthesis and Antioxidant Properties are Influenced by Wavelength of LED-Light in Arabidopsis Seedlings
애기장대의 포르피린 생합성과 항산화 기작에 대한 LED-광 파장의 효과
- Jin-Gil Kim, Sunyo Jung**
- Received January 26, 2015; Revised February 23, 2015; Accepted February 24, 2015;
- ABSTRACT
-
The effects of different light wavelengths on porphyrin biosynthesis and antioxidant mechanism were examined using different wavelengths from light emitting diodes (LED) with wavelengths of white LED (420-680 nm) as a control, blue LED (460-490 nm), green LED (520-550 nm), and red LED (620-650 nm). After 3 days of exposure to various wavelengths of LED lights, Arabidopsis seedlings treated with blue LED displayed significant increases in Mg-porphyrins including Mg-protoporphyrin IX (Mg-Proto IX), Mg-Proto IX methyl ester (ME), and protochlorophyllide, whereas red LED resulted in the lower levels of Mg-Proto IX and Mg-Proto IX ME, compared to white LED. Among various LEDs, blue LED induced the great increase in antioxidant property as indicated by increased transcript level of CatA, compared to white LED. Similarly, the blue-LED treatment greatly accumulated the amount of anthocyanin in Arabidopsis seedlings, compared to other wavelengths of LED lights. Our study demonstrates that different light wavelengths greatly influence plants’ physiological characteristics through altering porphyrin biosynthesis as well as antioxidant properties.
- Introduction
- Introduction
Light is an important environmental factor affecting plant development and critically regulates the plant growth by regulating the various morphological and physiological changes of plants (Drozdova et al., 2001; Li et al., 2009). Light-emitting diodes (LEDs) are solid-state, long-lived, durable sources of narrow-band light output that can be used in a range of horticultural and photobiological applications. A few studies investigated the effect of the light spectral quality on the plant growth and morphogenesis as well as physiological responses by using this technology (Li et al., 2009; Yorio et al., 2001).Many porphyrin-containing compounds are cofactors of apoproteins involved in photosynthesis (chlorophyll), respiration and oxygen metabolism (heme) (Wagner et al., 2004). Porphyrin biosynthesis starts at glutamyl-tRNAGlu, and the subsequently formed 5-aminolevulinicacid (ALA) is metabolized to form tetrapyrroles through a variety of reactions (Beale and Weinstein, 1990). Protoporphyrin IX (Proto IX) is directed to the Mg and Fe branches for chlorophyll and heme biosynthesis, respectively. Many intermediates in the porphyrin biosynthetic pathway, such as Proto IX and protochlorophyllide (Pchlide), react with molecular oxygen to form reactive oxygen species (ROS), which are harmful to cells and cause the peroxidation of membrane lipids (Back and Jung, 2010; Reinbothe et al., 1996). Tetrapyrrole synthesis and degradation are carefully adjusted to the cellular requirements, reflecting the different needs under varying environmental conditions (Reinbothe et al., 1996). However, little is known about the effect of light quality on modulation of porphyrin metabolism in plants grown under different LEDs.During stress, disruption of cellular homeostasis is accompanied by the generation of ROS, and the extent of stress-induced damage can be attenuated by the action of the cellular antioxidant systems, including ascorbate, glutathione, and enzymes capable of scavenging ROS (Foyer and Shigeoka, 2011; Suzuki et al., 2012). Ascorbate peroxidase (APX), peroxidase, and catalase detoxify H2O2 (Foyer and Shigeoka, 2011). Lipid-soluble antioxidants such as carotenoids protect the membranes and are particularly important in the chloroplast, where oxidizable polyunsaturated lipids abound (Ledford and Niyogi, 2005). Imbalance between production of ROS and their detoxification by enzymatic and non-enzymatic reactions causes oxidative stress in plants.To efficiently implement LEDs into commercial agricultural production practices, it is necessary to match the particular spectral characteristics of the light source with the photosynthetic and physiological requirements of the crop of interest. We attempted to control light quality for plant growth by using different wavelengths of LED-light sources. In this study, we investigated the influence of different LEDs on regulatory mechanism of porphyrin intermediates and their biosynthetic genes. Moreover, to shed light on increased antioxdative capacity driven by specific wavelength of light, we monitored the content of carotenoid, expression of an antioxidant gene, and anthocyanin accumulation under different LED lights.
- Materials and methods
- Materials and methods
- Plant growth and light treatment conditions
- Plant growth and light treatment conditions
Arabidopsis seeds were surface-sterilized in 70% ethanol for 5 min and in 2% sodium hypochlorite for 20 min, followed by several rounds of washing with sterile water. Seeds were sown on 0.5 Murashige and Skoog (MS) media, vernalized at 4°C for 3 days, and germinated at 25°C under dark condition for 5 days. Then, the Arabidopsis seedlings were grown under different (LED) for 3 days.Each light treatment was conducted in separately controlled chambers to be free from spectral interference among treatments. The LED array chambers were programmed to provide a 14 h light/10 h dark photoperiod at photosynthetic photon flux (PPF) maintained of approximately 150 μmol m-2 s-1. Arabidopsis plants were grown under four different light sources with broad spectrum-white LED (420-680 nm) as a control, blue LED (460-490 nm), green LED (520-550 nm), red LED (620-650 nm), and under constant dark condition.- Pigment extraction and analysis
- Pigment extraction and analysis
Porphyrins were extracted and analyzed following the method of Lermontova and Grimm (2000). Plant tissue (0.1 g) was grounded in 1.5 ml of methanol:acetone:0.1N NaOH (9:10:1, v/v/v), and the homogenate was centrifuged at 10,000 g for 10 min to remove cell debris and proteins. Porphyrins were separated by HPLC using a Novapak C18 column (4 μm particle size, 4.6 mm × 250 mm, Waters Chromatography, Milford, MA, USA) at a flow rate of 1 ml min–1. Porphyrins were eluted with a solvent system of 0.1 M ammonium phosphate (pH 5.8) and methanol. The column elute was monitored with a fluorescence detector (474, Waters) at excitation and emission wavelengths of 400 and 630 nm for Proto IX, and 415 and 595 nm for Mg-protoporphyrinIX (Mg-Proto IX), Mg-Proto IX methyl ester (ME), and protochlorophyllide (Pchlide.)For chlorophyll and carotenoid determination, plant tissue (0.1 g) was extracted with 100% acetone. The extracts were centrifuged at 10,000 g for 10 min, and the resulting supernatants were collected. Chlorophylls and carotenoids concentration were measured spectrophotometrically at 470, 644.8 and 661.6 nm and calculated by the method of Lichtenthaler (1987).Anthocyanins were extracted by homogenizing leaves (0.1 g) in a precooled mortar with 1 ml of acidified (1% HCl) methanol and maintained at 4°C for 4 h to avoid the degradation of chlorophylls whose products may interfere with the absorption of anthocyanins at 530 nm (Mancinelli et al., 1975). Particulates were removed by centrifugation at 10,000 g for 30 min. Anthocyanins were calculated by using Abs530–0.25 Abs657 to account for the contribution by chlorophylls.- RNA extraction, cDNA synthesis, and RT-PCR analysis
- RNA extraction, cDNA synthesis, and RT-PCR analysis
Total RNA was prepared from Arabidopsis seedlings using TRIZOL Reagent (Invitrogen), and 5 μg of RNA from each sample was used for the RT reaction (SuperScript III First- Strand Synthesis System, Invitrogen). Subsequently, 50 ng of cDNA was used for RT-PCR analysis. The specific primers were designed based on gene bank database. Amplification was performed in a thermal cycler (PTC-100, Biorad) using one step of 5 min at 95°C, 25–30 cycles of each 30s at 95°C, 30s at 60°C and 45s at 72°C, followed by final step of 5 min at 72°C. Actin was used as the internal control.
- Results and discussion
- Results and discussion
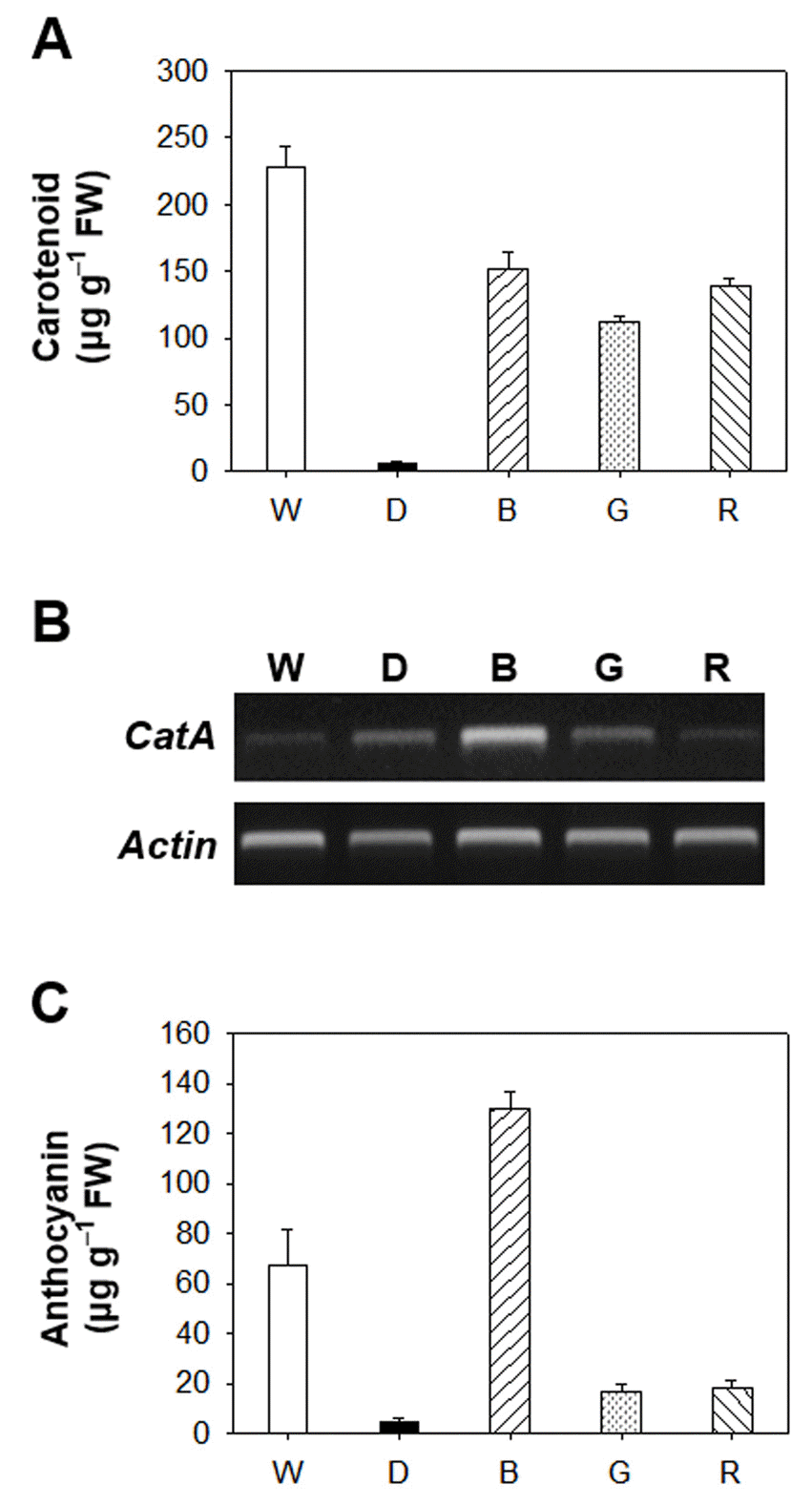
LEDs are durable, long-lived, and versatile sources for plant lighting that can be used for plant growth. The effects of different light wavelengths on photosynthetic pigment biosynthesis and antioxidant properties in Arabidopsis seedlings were examined using different wavelengths from LEDs with peak wavelengths of 470 nm (blue), 525 nm (green), 625 nm (red), and from white light emitting diodes as well as under constant dark condition. To explore the effect of different wavelengths of light on photosynthetic pigments, we quantitated the amount of total chlorophylls and porphyrin biosynthetic intermediates in Arabidopsis seedlings grown under various LED lights having different wavelengths. There was a considerable difference in the content of chlorophyll, which is the end product of porphyrin biosynthetic pathway, of Arabidopsis seedlings lightened with different LED sources. Compared to broadspectrum white LED, when Arabidopsis seedlings were treated with other LEDs, chlorophyll contents were greatly decreased in blue, green, and red LEDs (Fig. 1). The seedlings grown in constant dark condition showed a negligible level of chlorophylls. The chlorophyll a/b ratio slightly decreased in blue, green, and red LEDs, compared to that of white LED, indicating that narrow spectrum of LED significantly influenced total chlorophylls but not chlorophyll a/b ratio.Fig. 1.
The effect of different LED lights on the content of total chlorophyll (A) and chlorophyll a/b ratio (B) in Arabidopsis seedlings. The 5-day-old Arabidopsis seedlings were exposed to different LEDs with a 14 h light/10 h dark photoperiod or under constant dark condition for 3 days. W, broad spectrum-white LED (420-680 nm) as a control; B, blue LED (460-490 nm); G, green LED (520-550 nm); R, red LED (620-650 nm). The data represents the mean ± SE of three replicates.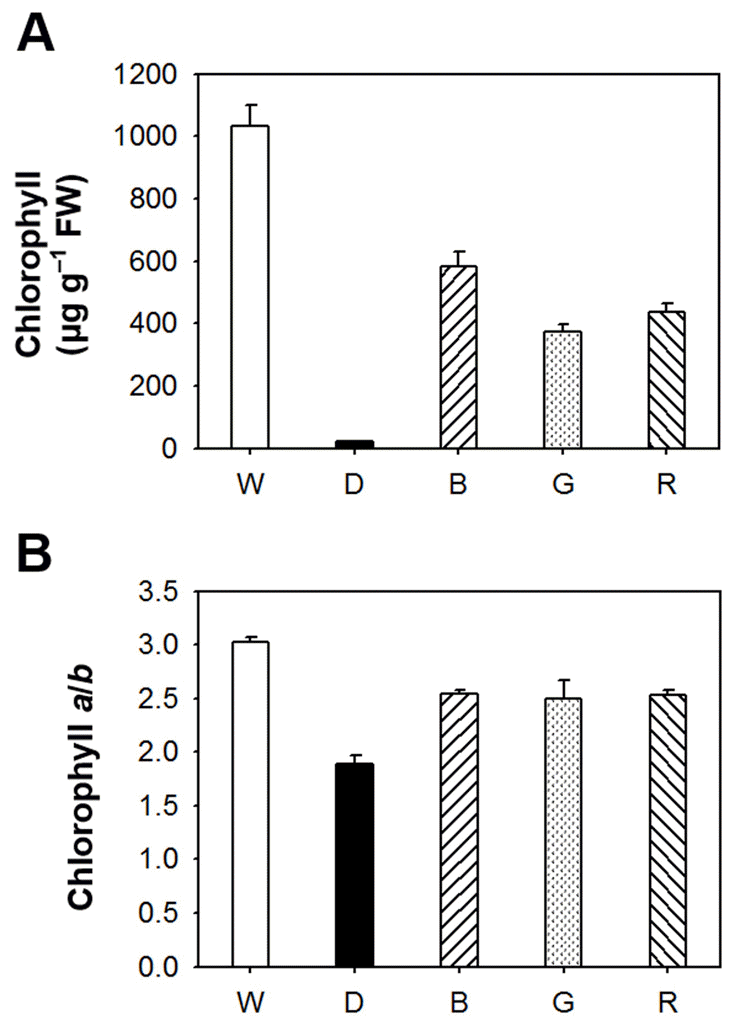 To assess the relationship between porphyrin biosynthesis and light wavelengths, we examined the effect of different LEDs on the regulation of porphyrin biosynthetic intermediates in Arabidopsis seedlings. A controlled flow of metabolites in the porphyrin biosynthetic pathway is essential for the fitness of photosynthetic organisms (Lermontova and Grimm, 2006), and plants suffer severe photodynamic damage if these control mechanisms are circumvented (Jung et al., 2008; Lermontova and Grimm, 2006). Interestingly, blue LED treatment greatly increased levels of Mg-tetrapyrroles including Mg-Proto IX, Mg-Proto IX ME, and Pchlide, but slightly decreased Proto IX level 3 days after the LED treatment (Fig. 2). The accumulation of Proto IX was the greatest under the dark condition. Green LED increased levels of Proto IX and Pchlide compared to white light, whereas red LED decreased levels of Proto IX, Mg-Proto IX and Mg-Proto IX ME. Our data provide evidence that narrow spectrum of each LED treatment has striking effects on the regulatory mechanism of porphyrin biosynthesis in Arabidopsis seedlings.
To assess the relationship between porphyrin biosynthesis and light wavelengths, we examined the effect of different LEDs on the regulation of porphyrin biosynthetic intermediates in Arabidopsis seedlings. A controlled flow of metabolites in the porphyrin biosynthetic pathway is essential for the fitness of photosynthetic organisms (Lermontova and Grimm, 2006), and plants suffer severe photodynamic damage if these control mechanisms are circumvented (Jung et al., 2008; Lermontova and Grimm, 2006). Interestingly, blue LED treatment greatly increased levels of Mg-tetrapyrroles including Mg-Proto IX, Mg-Proto IX ME, and Pchlide, but slightly decreased Proto IX level 3 days after the LED treatment (Fig. 2). The accumulation of Proto IX was the greatest under the dark condition. Green LED increased levels of Proto IX and Pchlide compared to white light, whereas red LED decreased levels of Proto IX, Mg-Proto IX and Mg-Proto IX ME. Our data provide evidence that narrow spectrum of each LED treatment has striking effects on the regulatory mechanism of porphyrin biosynthesis in Arabidopsis seedlings.Fig. 2.
The effect of different LED lights on the distribution of porphyrin intermediates. (A) Proto IX. (B) Mg-Proto IX. (C) Mg-Proto IX ME. (D) Pchlide. The plants were subjected to the same treatments as in Figure 1, and treatment notations are the same as in Figure 1. The data represent the mean ± S.E. of three replicates.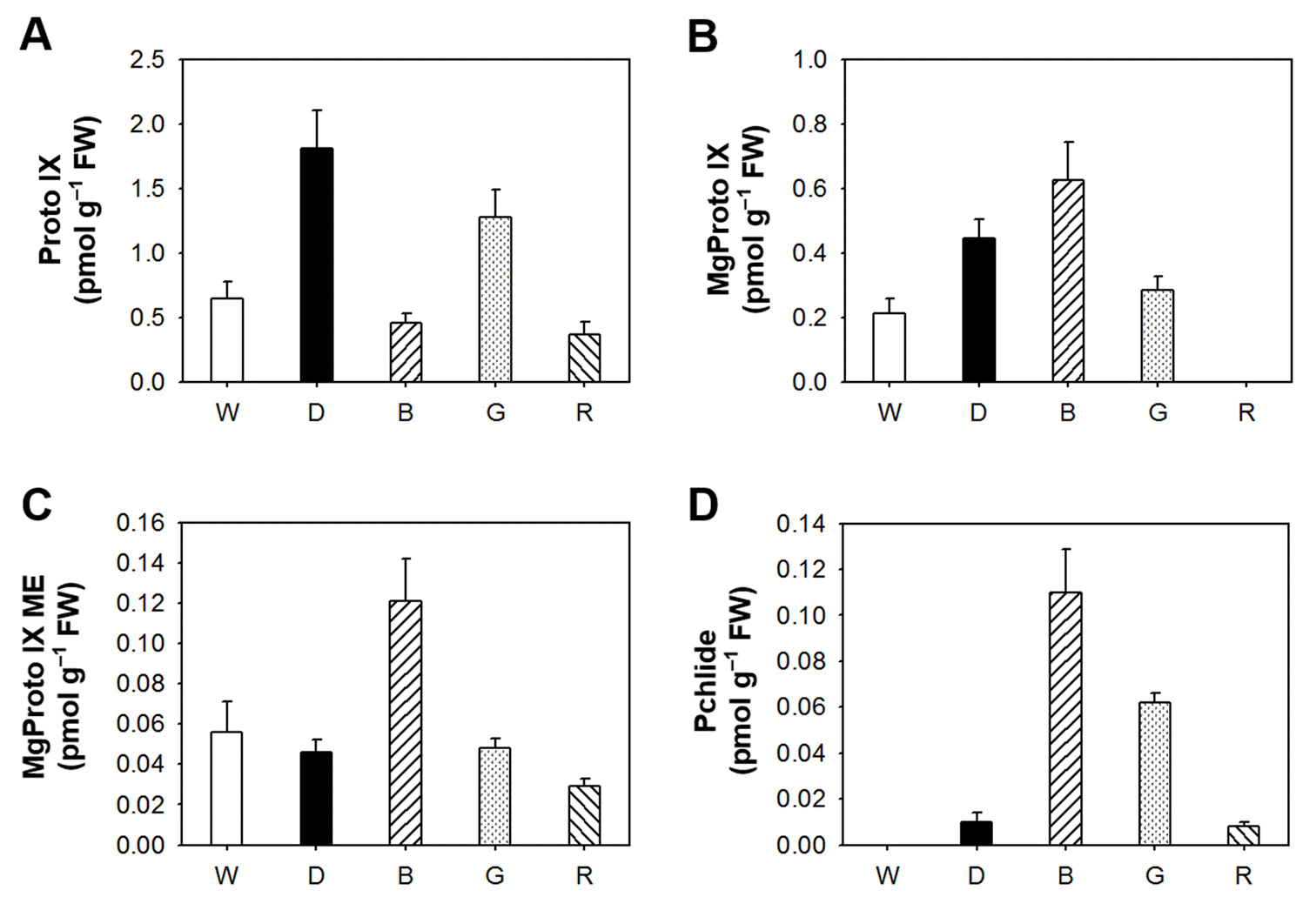 Expression levels of biosynthetic genes in the porphyrin pathway were examined in Arabidopsis seedlings grown under different LEDs. During prolonged blue LED, transcript levels of HEMA1 involving ALA-synthesizing activity and PPO1 encoding the protoporphyrinogen oxidase (PPO) which produces Proto IX were up-regulated, compared to those of white LED, whereas transcript levels of FC2, which encodes the plastidic isoform of Fe-chelatase, and CHLH were similar to those of white LED. However, both green and red LEDs decreased transcript levels of all porphyrin biosynthetic genes examined in this study (Fig. 3). In addition to its enzymatic functions as a subunit of Mg-chelatase, CHLH has a key function in mediating plastid-to-nucleus retrograde signaling by controlling the metabolism of the porphyrin signal MgProto IX or by sensing the signal (Nott et al., 2006; Strand et al., 2003). PPO might play an important role as a key regulatory enzyme when the porphyrin biosynthetic pathway is deregulated under blue LED. Taken together, we conclude that Proto IX and Mgporphyrin intermediate levels are all greatly influenced in Arabidopsis seedlings following different LED treatments, although it remains necessary to explain how a specific LED wavelength leads to the altered porphyrin biosynthesis.
Expression levels of biosynthetic genes in the porphyrin pathway were examined in Arabidopsis seedlings grown under different LEDs. During prolonged blue LED, transcript levels of HEMA1 involving ALA-synthesizing activity and PPO1 encoding the protoporphyrinogen oxidase (PPO) which produces Proto IX were up-regulated, compared to those of white LED, whereas transcript levels of FC2, which encodes the plastidic isoform of Fe-chelatase, and CHLH were similar to those of white LED. However, both green and red LEDs decreased transcript levels of all porphyrin biosynthetic genes examined in this study (Fig. 3). In addition to its enzymatic functions as a subunit of Mg-chelatase, CHLH has a key function in mediating plastid-to-nucleus retrograde signaling by controlling the metabolism of the porphyrin signal MgProto IX or by sensing the signal (Nott et al., 2006; Strand et al., 2003). PPO might play an important role as a key regulatory enzyme when the porphyrin biosynthetic pathway is deregulated under blue LED. Taken together, we conclude that Proto IX and Mgporphyrin intermediate levels are all greatly influenced in Arabidopsis seedlings following different LED treatments, although it remains necessary to explain how a specific LED wavelength leads to the altered porphyrin biosynthesis.Fig. 3.
LED-induced changes in the expression of genes encoding the porphyrin pathway enzymes. The plants were subjected to the same treatments as in Fig. 1. Treatment notations are the same as in Fig. 1. Total RNAs were purified from plants and reverse transcribed. The resultant cDNAs were used as templates for RT-PCR using Actin as an internal control.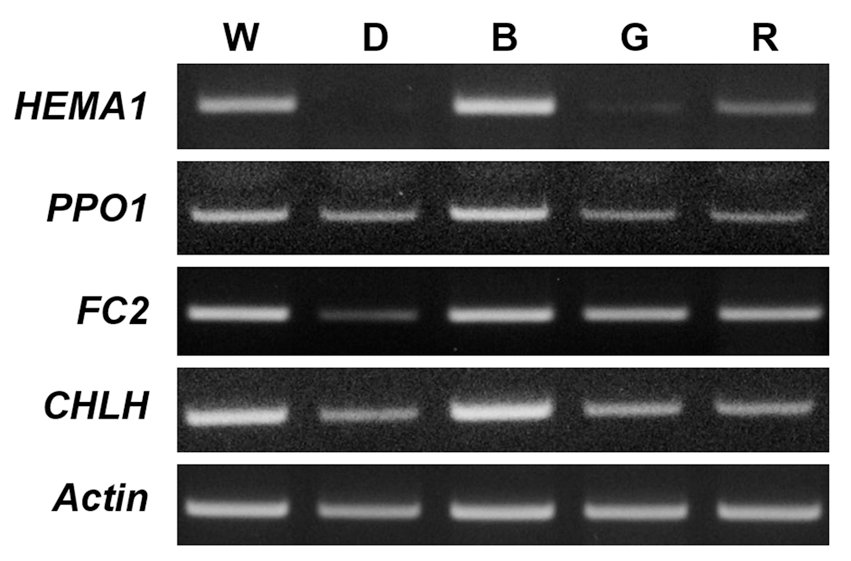 Environmental stresses including light stress control metabolites of the porphyrin biosynthetic pathway through their scavenging to cope with excited-state dynamics of porphyrin in the cell (Kim et al., 2011; Phung et al., 2011). The altered dynamics of these photosensitizing porphyrins may lead to an altered redox state of the plastid. In plants, antioxidant defense systems include various antioxidants, such as carotenoids, tocopherol, flavonoids, ascorbate and phenolic compounds, which play important roles in protection from photooxidative damage (Ashry and Mohamed, 2011; Samuoliene et al., 2010). In response to exposure of Arabidopsis seedlings to blue, green, or red LEDs as well as constant dark condition, the contents of total carotenoids greatly decreased compared to white LED (Fig. 4A). The lack of photoprotective carotenoids in Arabidopsis grown under blue, green, or red LEDs may result in phooxidative stress in those plants. We also assessed the transcript level of CatA encoding catalase enzyme of Arabidopsis seedlings grown under different LEDs (Fig. 4B). Blue LED greatly accumulated transcript level of CatA in Arabidopsis seedlings compared to white LED, whereas no significant differences between other LEDs and white LED were found for CatA transcript.The induction of anthocyanin is known to be affected by light quality (Mancinelli et al., 1975). High concentrations of ultraviolet light have been associated with induction of anthocyanin (Krizek et al., 1998). In our study, blue LED greatly accumulated the content of anthocyanin compared to white LED (Fig. 4C). In contrast, red and green LED lights significantly decreased the amount of anthocyanin, compared to white LED conditions. In higher plants, blue-light is mainly perceived by cryptochromes and phototropins, which subsequently orchestrates phototropism, chloroplast relocation, stomatal opening, rapid inhibition of hypocotyl elongation and leaf expansion (Shimazaki et al., 2007; Yorio et al., 2001). The accumulation of anthocyanin under blue LED may be due to a higher energy level of blue light and appears to be regulated by cryptochrome, however, relevant mechanisms are largely unknown.Our study demonstrate that a narrow spectrum of different LEDs significantly influence the levels of chlorophylls, porphyrin intermediates and their biosynthetic genes as well as antioxidant properties in Arabidopsis seedlings. Particularly, blue LED is highly efficient to protect plants from oxidative stress, as indicated by the increased production of antioxidant mechanisms, including anthocyanin and transcript level of antioxidant gene CatA. Nevertheless, it must be prompted which signaling pathway is activated by blue light to gain more insight into the light wavelength-dependent physiological modifications in plants. This may act through altered porphyrin biosynthesis as well as antioxidant mechanisms. The characterization of light quality on plant growth and development of high-output LEDs will provide the opportunity to develop the unique characteristics of LEDs as tools for enhancing yield, extend seasons, and improve quality of plants.
Environmental stresses including light stress control metabolites of the porphyrin biosynthetic pathway through their scavenging to cope with excited-state dynamics of porphyrin in the cell (Kim et al., 2011; Phung et al., 2011). The altered dynamics of these photosensitizing porphyrins may lead to an altered redox state of the plastid. In plants, antioxidant defense systems include various antioxidants, such as carotenoids, tocopherol, flavonoids, ascorbate and phenolic compounds, which play important roles in protection from photooxidative damage (Ashry and Mohamed, 2011; Samuoliene et al., 2010). In response to exposure of Arabidopsis seedlings to blue, green, or red LEDs as well as constant dark condition, the contents of total carotenoids greatly decreased compared to white LED (Fig. 4A). The lack of photoprotective carotenoids in Arabidopsis grown under blue, green, or red LEDs may result in phooxidative stress in those plants. We also assessed the transcript level of CatA encoding catalase enzyme of Arabidopsis seedlings grown under different LEDs (Fig. 4B). Blue LED greatly accumulated transcript level of CatA in Arabidopsis seedlings compared to white LED, whereas no significant differences between other LEDs and white LED were found for CatA transcript.The induction of anthocyanin is known to be affected by light quality (Mancinelli et al., 1975). High concentrations of ultraviolet light have been associated with induction of anthocyanin (Krizek et al., 1998). In our study, blue LED greatly accumulated the content of anthocyanin compared to white LED (Fig. 4C). In contrast, red and green LED lights significantly decreased the amount of anthocyanin, compared to white LED conditions. In higher plants, blue-light is mainly perceived by cryptochromes and phototropins, which subsequently orchestrates phototropism, chloroplast relocation, stomatal opening, rapid inhibition of hypocotyl elongation and leaf expansion (Shimazaki et al., 2007; Yorio et al., 2001). The accumulation of anthocyanin under blue LED may be due to a higher energy level of blue light and appears to be regulated by cryptochrome, however, relevant mechanisms are largely unknown.Our study demonstrate that a narrow spectrum of different LEDs significantly influence the levels of chlorophylls, porphyrin intermediates and their biosynthetic genes as well as antioxidant properties in Arabidopsis seedlings. Particularly, blue LED is highly efficient to protect plants from oxidative stress, as indicated by the increased production of antioxidant mechanisms, including anthocyanin and transcript level of antioxidant gene CatA. Nevertheless, it must be prompted which signaling pathway is activated by blue light to gain more insight into the light wavelength-dependent physiological modifications in plants. This may act through altered porphyrin biosynthesis as well as antioxidant mechanisms. The characterization of light quality on plant growth and development of high-output LEDs will provide the opportunity to develop the unique characteristics of LEDs as tools for enhancing yield, extend seasons, and improve quality of plants.
- 적요
- 적요
이번 연구에서는 white LED(420-680nm), blue LED(460- 490nm), green LED(520-550nm), red LED(620-650nm)를 포 함하는 서로 다른 빛의 파장을 가지는 light emitting diodes(LED) 가 포르피린 생합성과 항산화 기작에 미치는 영향을 조사하였다. 다양한 파장의 LED 광을 3일간 식물체에 조사하였을 때, white LED와 비교하여 blue LED로 처리된 애기장대 식물은 Mgprotoporphyrin IX (Mg-Proto IX), Mg-Proto IX methyl ester (ME), and protochlorophyllide을 포함하는 Mg-porphyrins을 현 저하게 증가시킨 반면에 red LED는 Mg-Proto IX과 Mg-Proto IX ME의 감소를 보여 주었다. 다양한 LED 중에서 blue LED가 white LED와 비교하여 CatA의 유전자 발현의 증가에 의해 표시 되는 항산화 작용의 증가를 크게 유도하였다. 또한, blue LED는 다 른 LED 광의 파장과 비교하여 애기장대의 anthocyanin 수준을 크 게 축적하였다. 이번 연구는 서로 다른 빛의 파장이 항산화 작용뿐 만 아니라 포르피린 생합성 과정을 변화시킴에 의해 식물의 생리학 적 특성에 큰 영향을 미침을 보여 준다.
- REFERENCES
- REFERENCES
- 1. Ashry, N.A, H.I Mohamed. Impact of secondary metabolites and related enzymes in flax resistance and or susceptibility to powdery mildew. World J. Agric. Sci 2011;7:78-85.2. Back, K, S Jung. The lack of plastidal transit sequence cannot override the targeting capacity of Bradyrhizobium japonicum d-aminolevulinic acid synthase in transgenic rice. Biol. Plant 2010;54:279-284.
[Article]3. Daily, H.A, S.I Beale, J.D Weinstein. Tetrapyrrole metabolism in photosynthetic organisms Biosynthesis of Heme and Chlorophyll 1990;New York USA. McGraw-Hill; 287-391.4. Drozdova, I.S, V.V Bondar, N.G Bukhov, A.A Kotov, L.M Kotova, S.N Maevskaya, A.T Mokronosov. Effects of light spectral quality on morphogenesis and source-sink relations in radish plants. Russ. J. Plant Physiol 2001;48:415-420.
[Article]5. Foyer, C.H, S Shigeoka. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 2011;155:93-100.
[Article] [PubMed]6. Jung, S, H.J Lee, Y Lee, K Kang, Y.S Kim, B Grimm, K Back. Toxic tetrapyrrole accumulation in protoporphyrinogen IX oxidase- overexpressing transgenic rice plants. Plant Mol. Biol 2008;67:535-546.
[Article] [PubMed]7. Kim, J.G, K Back, H.Y Lee, H.J Lee, T.H Phung, B Grimm, S Jung. Increased expression of Fe-chelatase leads to increased metabolic flux into heme and confers protection against photodynamically induced oxidative stress. Plant Mol. Biol 2014;86:271-287.
[Article] [PubMed]8. Krizek, D.T, S.J Britz, R.M Mirecki. Inhibitory effects of ambient levels of solar UV-A and UV-B radiation on growth of cv. New Red Fire lettuce. Physiol. Plant 1998;103:1-7.9. Ledford, H.K, K.K Niyogi. Singlet oxygen and photo-oxidative stress management in plants and algae. Plant Cell Environ 2005;28:1037-1045.
[Article]10. Lermontova, I, B Grimm. Overexpression of plastidic protoporphyrinogen IX oxidase leads to resistance to the diphenyl-ether herbicide acifluorfen. Plant Physiol 2000;122:75-83.
[Article] [PubMed] [PMC]11. Lermontova, I, B Grimm. Reduced activity of plastid protoporphyrinogen oxidase causes attenuated photodynamic damage during high-light compared to low-light exposure. Plant J 2006;48:499-510.
[Article] [PubMed]12. Li, Q, C Kubota. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot 2009;67:59-64.
[Article]13. Lichtenthaler, H.K Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 1987;148:350-382.
[Article]14. Mancinelli, A.L, C.P.H Yang, P Lindquist, O.R Anderson, I Rabino. Photocontrol of anthocyanin synthesis III The action of streptomycin on the synthesis of chlorophyll and anthocyanin. Plant Physiol 1975;55:251-257.
[Article] [PubMed] [PMC]15. Nott, A, H.S Jung, S Koussevitzky, J Chory. Plastidto- nucleus retrograde signaling. Annu. Rev. Plant Biol 2006;57:739-759.
[Article] [PubMed]16. Phung, T.H, H.I Jung, J.H Park, J.G Kim, K Back, S Jung. Porphyrin biosynthesis control under water stress: sustained porphyrin status correlates with drought tolerance in transgenic rice. Plant Physiol 2011;157:1746-1764.
[Article] [PubMed] [PMC]17. Reinbothe, S, C Reinbothe, K Apel, N Lebedev. Evolution of chlorophyll biosynthesis–The challenge to survive photooxidation. Cell 1996;86:703-705.
[Article] [PubMed]18. Samuoliene, G, A Brazaityte, A Urbonaviciute, G Sabajeviene, P Duchovskis. The effect of red and blue light component on the growth and development of frigo strawberries. Zemdirbyste 2010;97:99-104.19. Shimazaki, K.I, M Doi, S.M Assmann, T Kinoshita. Light regulation of stomatal movement. Annu. Rev. Plant Biol 2007;58:219-247.
[Article]20. Strand, A, T Asami, J Alonso, J.R Ecker, J Chory. Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrin IX. Nature 2003;421:79-83.
[Article] [PubMed]21. Suzuki, N, S Koussevitzky, R Mittler, G Miller. ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ 2012;35:259-270.
[Article] [PubMed]22. Wagner, D, D Przybyla, R Op den Camp, C Kim, F Landgraf, K.P Lee, M Würsch, C Laloi, M Nater, E Hideg, K Apel. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 2004;306:1183-1185.
[Article] [PubMed]23. Yorio, N.C, G.D Goins, H.R Kagie, R.M Wheeler, J.C Sager. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. Hort. Sci 2001;36:380-383.
[Article]