Effects of Green Walls on Prefrontal Cerebral Hemodynamics in Hospital Workers
Article information
Abstract
Background and objective
Artificial environments have been known to negatively affect neuropsychological activities. Healthcare workers who are under high psychological and physiological stress often spend long hours at a time. Recently, many studies have been conducted to reveal the healing effects of natural environments, but few studies have been conducted on healthcare workers. Therefore, this study aims to investigate the effect of green walls in medical facilities on the hemodynamics of the prefrontal cortex in healthcare workers.
Methods
Eleven healthy adults working in a medical institution participated in the experiment, in which they rested for 10 minutes in a room with green walls and in a control room. To compare the physiological and psychological changes between the green wall room and the control room, we used various measurements, such as near-infrared spectroscopy in the prefrontal area (NIRS), heart rate variability (HRV), blood pressure (BP), and pulse rate (PR). Psychological tests were also conducted using the Profile of Mood States (POMS), the State-Trait Anxiety Inventory (STAI), the Perceived Restorativeness Scale (PRS), and the Zucherman Inventory of Personal Reaction Scale (ZIPERS).
Results
The oxyhemoglobin concentration in the frontal lobe significantly decreased on both the left and right sides in the green wall room compared to the control room. Green walls significantly activated the parasympathetic nervous system and inhibited the sympathetic nervous system compared to the control room. In addition, psychological reactions increased positive emotions and decreased negative emotions.
Conclusion
Green walls in medical facilities could be an effective way to promote physiological relaxation and health by reducing physiological hemodynamics in the prefrontal cortex evoked by psychological stress in healthcare workers. This study implies that green walls can be used as an effective means of stress reduction and relaxation.
Introduction
The prolonged COVID-19 pandemic has resulted in high workloads for medical workers, which may cause cumulative mental and physical stress and serve as risk factors for health (Wu et al., 2020; Tan et al., 2020). Because healthcare workers spend a long time in closed indoor spaces and have little chance to be in contact with the outdoor natural environment, there is an urgent need for stress relief through adequate rest, but there are insufficient places to relieve stress within hospitals and medical facilities (Chen et al., 2020). In fact, according to a study on healthcare workers, approximately 65% were suffering from severe stress (Park and Lee, 2022). Moreover, a study of non-medical staff working in medical institutions among healthcare workers reported that non-medical staff experienced 1.85 times more anxiety symptoms than medical personnel (Tan et al., 2020). Such accumulation of fatigue and stress may deteriorate the function of the frontal lobe and develop into serious health problems, such as cardiovascular diseases, depression, and cognitive impairment (Dedovic et al., 2009; Castaldo et al., 2015).
Many researchers have suggested that a natural environment can be an alternative for stress relief and health promotion (Hansen et al., 2017; Tsunetsugu et al., 2010; Miyazaki, 2018). According to study results verifying the therapeutic effect of a natural environment on humans, being exposed to this environment helps reduce negative emotions, such as anxiety, fatigue, and depression, and is effective in relieving emotional stress by inducing positive emotions (Kang et al., 2020; Kim et al., 2022; Kim et al., 2021; Lee et al., 2012). Moreover, studies using objective indicators have investigated cerebral function (Norwood et al., 2019), heart rate variability (Park et al., 2014; Song, 2015), salivary cortisol (Park et al., 2010; Lee et al., 2011; Ochiai, 2015), and NK cells activation (Li et al., 2007), implying that natural environments induce physiological relaxation. Recently, there has been active research on different brain responses to various environments. A natural environment reduced cerebral stress levels by lowering amygdala activity (Lederbogen et al., 2011), and the act of viewing forests (Park et al., 2007) or gardens (Lee, 2017) turned out to have a positive effect on the hemodynamic change of the frontal lobe. Experiencing nature in indoor spaces has also proven effective in relieving stress (Kim et al., 2021).
Many of hospitals do not have enough outdoor rest areas, which is why attempts have been made to implement spaces to relieve stress by creating gardens indoors or on building rooftops. Some studies have reported how natural elements in indoor hospital spaces affect patients (Park and Mattson, 2008; Yar and Kazemi, 2020). Studies on healthcare workers have shown that looking at plant images (Nejati et al., 2016) and real flowers (Komatsu et al., 2013) helps workers recover vigor. However, there are insufficient studies in Korea on the effectiveness of green walls in hospitals on healthcare workers. Therefore, this study aims to investigate the effects of green walls made with plants on the psychological stress of workers in medical facilities and to quantitatively analyze the functional changes to their frontal lobes.
Research Methods
Research site and subjects
This study quantitatively derives the physiological and psychological changes of workers in medical institutions while relaxing in a rest area made of green plants. The field experiment was conducted at C Medical Center in Cheonan city, Korea. Green walls of approximately 21.0 m2 were created in the size of 6.0 m wide and 3.5 m high within the medical center. Eleven plant species were used: Rhapis excelsa, Davallia bullata, Dracaena fragrans ‘Victoria’, Lime (Neon) Pothos, Trachelospermum jasminoides, Hedera helix ‘Smithii,’ Ardisia pusilla ‘Variegata,’ Ardisia japonica, Chamaedorea elegans, Hoya carnosa, and Ardisia pusilla. These individual plants have an average height of 15 cm – 30 cm and were installed by fixing the separate pots on the wall so that the entire space looked like a green wall from the outside. The plants were planted in 41 – 45 15 cm × 20 cm pots for each plant species in clusters. The control area was a common room space in the building that had cement walls finished with white paint. The size and structure were similar to those of the green wall, and the floor finish was also the same (Fig. 1). The participants were recruited for 15 days. They were chosen from a list of individuals who applied through online/offline notices following consultation with C Medical Center. They comprised 11 healthy male and female adults in their 20s – 50s (mean age 43.5 ± 7.1 years old) working in the medical institution and with no medical history of cardiovascular or mental diseases. To observe the physiological and psychological changes that might occur depending on where they were resting, we used various measurements, such as oxyhemoglobin of the prefrontal cortex (Oxy-Hb), heart rate variability (HRV), blood pressure (BP), pulse rate (PR), and psychometrics. Before starting the experiment, we provided informed explanation of the study and obtained the written consent. We attached the HRV sensor on the left side of their chests and the NIRS device on their foreheads about 10 mm – 20 mm above the eyebrows and moved them to the green wall room or the control room. As in previous studies, (Henning et al., 1997; Chavko and McCarron, 2006; Mullen et al., 2012), we offset the effect of prior stimulus by having the subjects relax for 3 minutes in each space and then rest facing the front for 10 minutes (based on the usual break time for healthcare workers). They rested once each in the green wall room and the control room so that physiological and psychological data could be collected, and the order of the experiment was random. Moreover, since the physical movements of the subjects during the experiment may affect physiological data, wheelchairs were used to move subjects from one space to the other. This study was approved by the Public Institutional Review Board (P01-202110-12-003).
Physiological measurement index
Frontal lobe function (Oxy-Hb)
The near-infrared spectroscopy (NIRS) device used to measure changes in frontal lobe function due to rest in different spaces is a tool that measures the hemodynamic state of living tissues using light absorbance in a wavelength between 650 nm and 1,000 nm. This device can identify changes in the oxyhemoglobin and deoxyhemoglobin concentrations of the frontal lobe, which is one of the central nervous system activity indices in charge of cognitive function (Ohmae et al., 2006). This study analyzed oxyhemoglobin, including oxygen, and used NIRS Lite (OBELAB, Korea) for measurement. NIRS Lite is comprised of a total of 15 channels: seven left channels to measure the hemodynamic change of the left prefrontal cortex (CH9 - CH15), seven right channels to measure the hemodynamic change of the right prefrontal cortex (CH1 - CH7), and one central channel (CH8).
Autonomic function
Heart rate variability (HRV), which can evaluate autonomic function, measures parasympathetic and sympathetic activity by converting R waves and the fluctuations in between into frequencies. In this study, high frequency (HF: 0.15 – 0.40 Hz), low frequency (LF: 0.04 – 0.15 Hz), and the ratio of the two frequencies (LF/HF) were extracted (Malik, 1996) using My Beat (UNION TOOL, Japan). HF, reflecting parasympathetic activity, is activated in a relaxed state; LF/HF, reflecting sympathetic activity, is activated in a tense state (Cacioppo et al., 1994). In addition, BP and PR, which can detect autonomic changes, were measured. We measured BP and PR twice, each from the right upper arm of the subjects, using a BP monitor HEM-1000 (OMRON, Japan), and analyzed the average.
Psychometric index
Perceived Restorativeness Scale (PRS)
The Perceived Restorativeness Scale (PRS) was used to identify how the subjects perceived the characteristics of each space as a restorative environment (Hartig et al., 1997). The PRS subdivides the four domains of the Attention Restoration Theory (ART) by Kaplan and Kaplan (1989) ‘being away,’ ‘fascination,’ ‘extent,’ and ‘compatibility’ into 16 items are rated on a 10-point scale. There are 16 items, with a higher total score indicating that the space has elements of a restorative environment and leads to a higher psychological restoration effect. Cronbach’s α of the index used in this study was 0.964.
Zucherman inventory of personal reaction scale (ZIPERS)
The Zucherman Inventory of Personal Reaction Scale (ZIPERS), which was used to identify the changes in individual psychological stress levels caused by resting in different environments, is comprised of five domains: ‘positive affect,’ ‘attentiveness,’ ‘fear,’ ‘anger,’ and ‘sadness,’ with 12 items rated on a 5-point Likert scale (Zuckerman, 1977). Higher scores for each domain indicate higher psychological levels, and Cronbach’s α was 0.658.
Profile of Mood States (POMS)
The Profile of Mood States (POMS) was used to measure mood states while resting in the green wall room and the control room (Mcnair et al., 1992; Yeun and Shin-park, 2006). This tool is classified into six domains: ‘tension-anxiety (T-A),’ ‘anger-hostility (A-H),’ ‘depression (D),’ ‘vigor (V),’ ‘fatigue (F),’ and ‘confusion (C).’ Each can be scored (McNair and Lorr, 1964; McNair et al., 1992), with a total of 30 items rated on a 5-point Likert scale. This study used only three domains: ‘depression (D),’ ‘vigor (V),’ and ‘fatigue (F),’ and Cronbach’s α was 0.640.
State-Trait Anxiety Inventory (STAI)
The State-Trait Anxiety Inventory (STAI-X-1) was used to objectively measure and score individuals’ perceived anxiety levels (Spielberger, 1970; Kim and Shin, 1978). A total of 20 items were rated on a 4-point Likert scale, with higher total scores indicating higher state-trait anxiety levels. Cronbach’s α was 0.884.
Analytical tool
We compared the physiological and psychological data of 11 subjects resting in the green wall room and the control room. This study analyzed 15 NIRS channels for 10 minutes (600 seconds), the left and right channels excluding the central channel. For time series analysis, we divided the 600-second data into 60-second units and calculated the average. For physiological and psychological data, Wilcoxon signed-rank test was conducted using IBM statistics SPSS 21.0 (IBM, Armonk, NY). The results were indicated in mean ± SD, and the statistical significance level was set at p < .05. For the analysis of HRV data, eight samples were used, excluding error data.
Results and Discussion
Results of physiological measurement
Changes in frontal lobe function (Oxy-Hb)
While resting in the green wall room and control room, there were significant changes in the oxyhemoglobin concentration of the cerebral prefrontal cortex. As we compared the means of the blood flow in the prefrontal cortex for 600 seconds each, we found that the oxyhemoglobin concentration was significantly lower in the green wall room compared to the control room (Control, −0.165 ± 0.134 μmol; Green wall, −0.613 ± 0.190 μmol; p < .001) (Fig. 2).
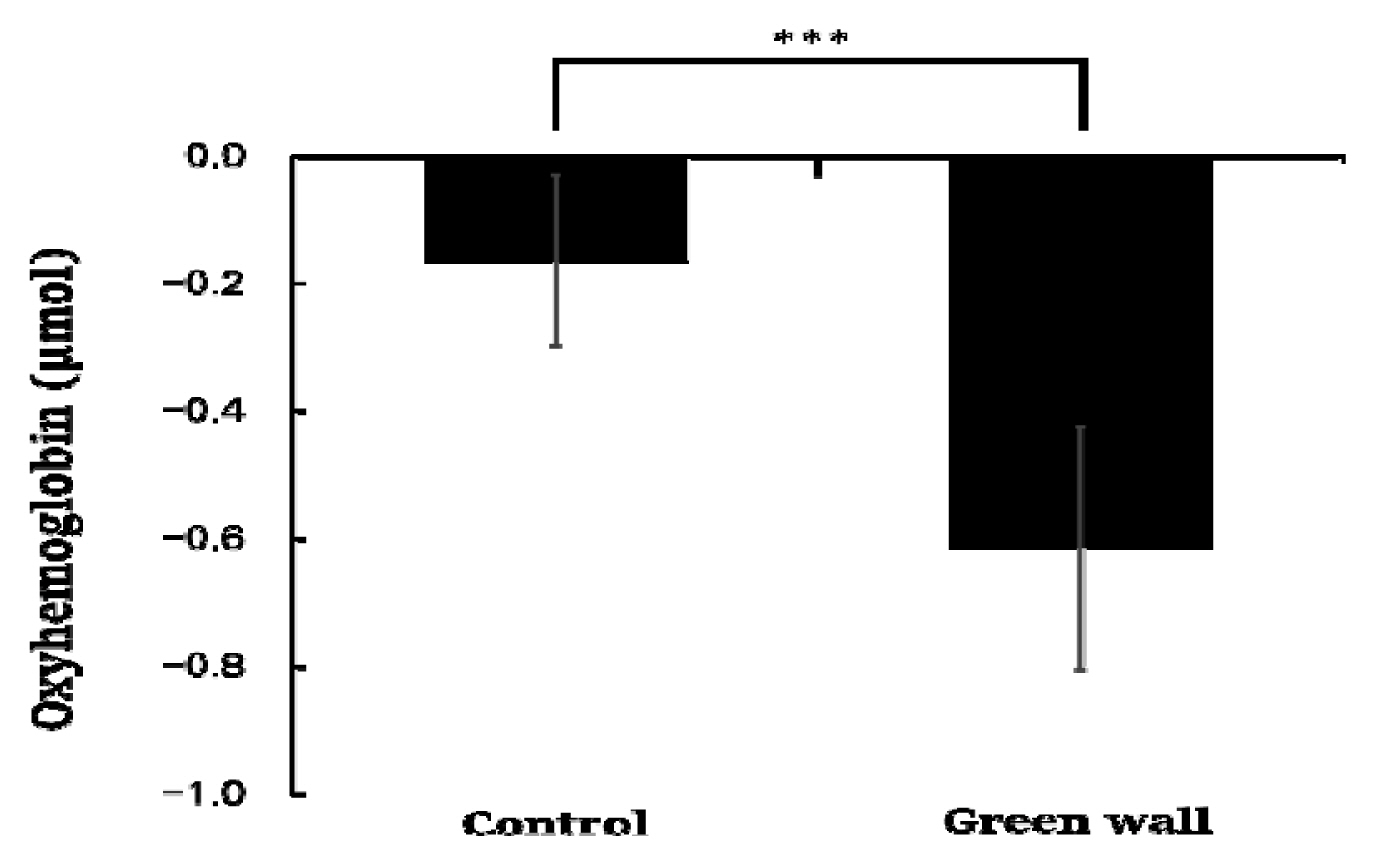
Comparison of the mean oxyhemoglobin concentration of the control and green walls for the entire prefrontal cortex. N = 11; ***, p < .001; Wilcoxon signed-rank test.
We calculated the mean by dividing the oxyhemoglobin concentration in the prefrontal cortex into 60 seconds and discovered that the concentration was generally lower in the green wall room. As we compared the decrease in blood flow every 60 seconds, we found that there was a more significant decrease in the green wall room compared to the control room in all sections except 60 to 120 seconds (Control, −0.190 ± 0.120 μmol; Green wall, −0.635 ± 0.201 μmol; p < .001) and 480 to 540 seconds (Control, −0.173 ± 0.124 μmol; Green wall, −0.669 ± 0.097 μmol; p < .001). In particular, there was an even more significant decrease in 300 to 360 seconds (Control, −0.211 ± 0.081 μmol; Green wall, −0.727 ± 0.288 μmol; p < .001) and 420 to 480 seconds (Control, −0.088 ± 0.112 μmol; Green wall, −0.654 ± 0.062 μmol; p < .001) (Fig. 3).
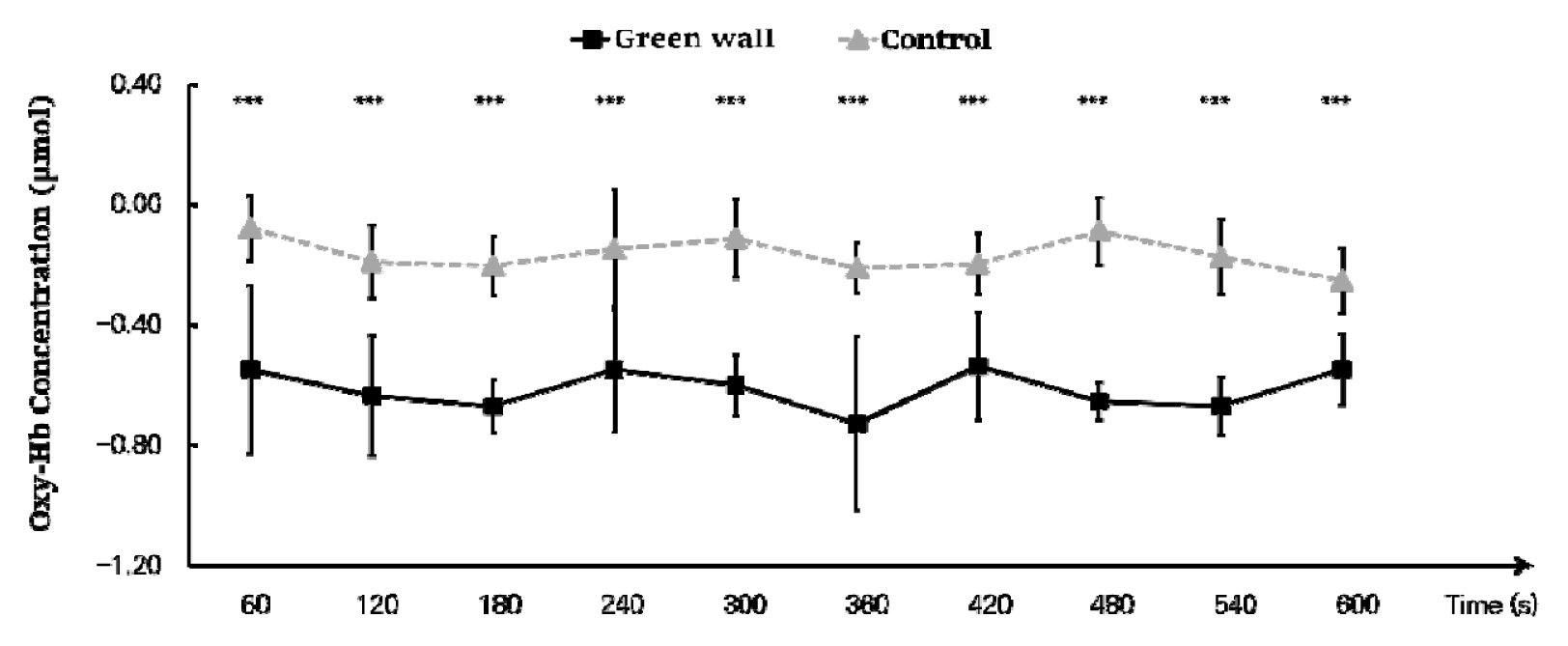
Comparison of oxyhemoglobin concentrations of control and green walls for the entire prefrontal cortex for 60 seconds. N=11; ***, p < .001; Wilcoxon signed-rank test.
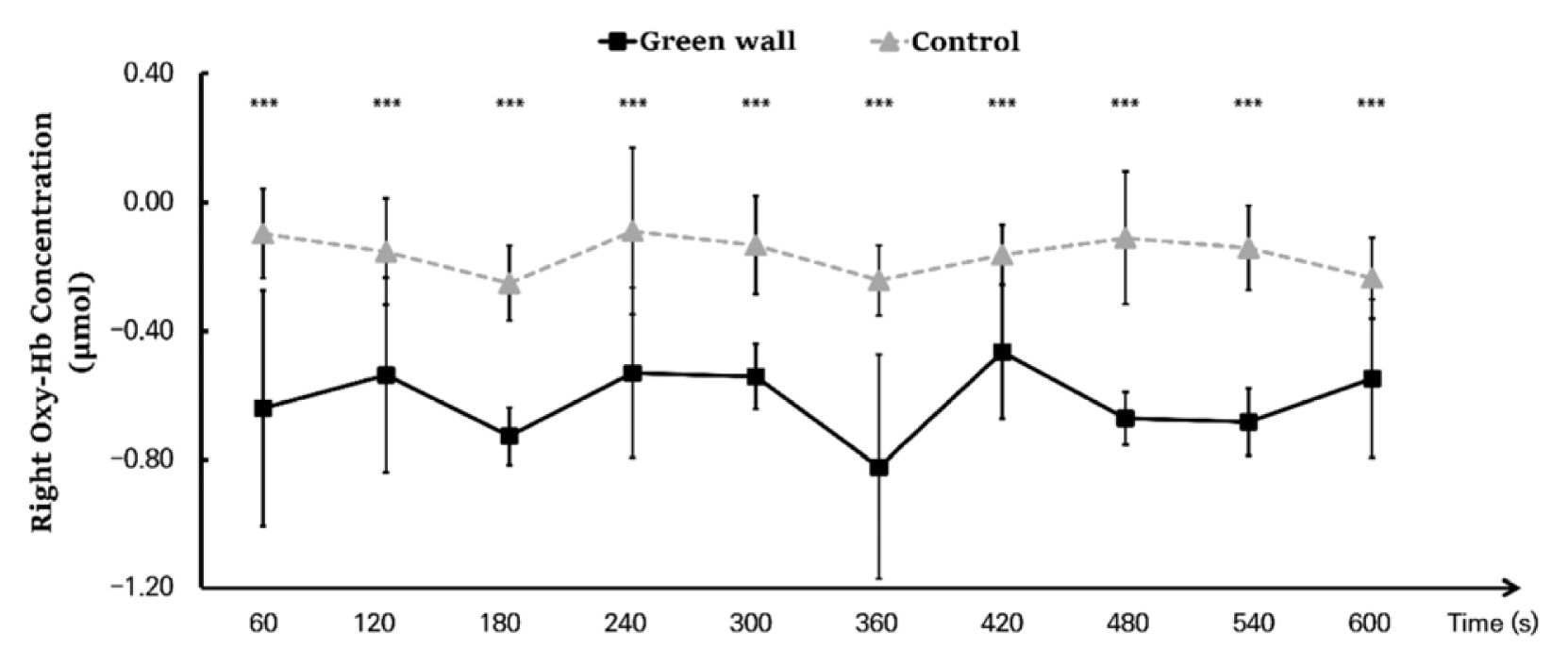
We compared the means for 600 seconds by dividing the cortex into the left prefrontal cortex and right prefrontal cortex. We discovered that, in the left prefrontal cortex, oxyhemoglobin concentration while resting in the green wall room was significantly lower than in the control room (Control, −0.158 ± 0.144 μmol; Green wall, −0.634 ± 0.184 μmol; p < .001). Concentration in the right prefrontal cortex was also significantly lower in the green wall room than in the control room (Control, −0.162 ± 0.167 μmol; Green wall, −0.616 ± 0.259 μmol; p < .001) (Fig. 4).
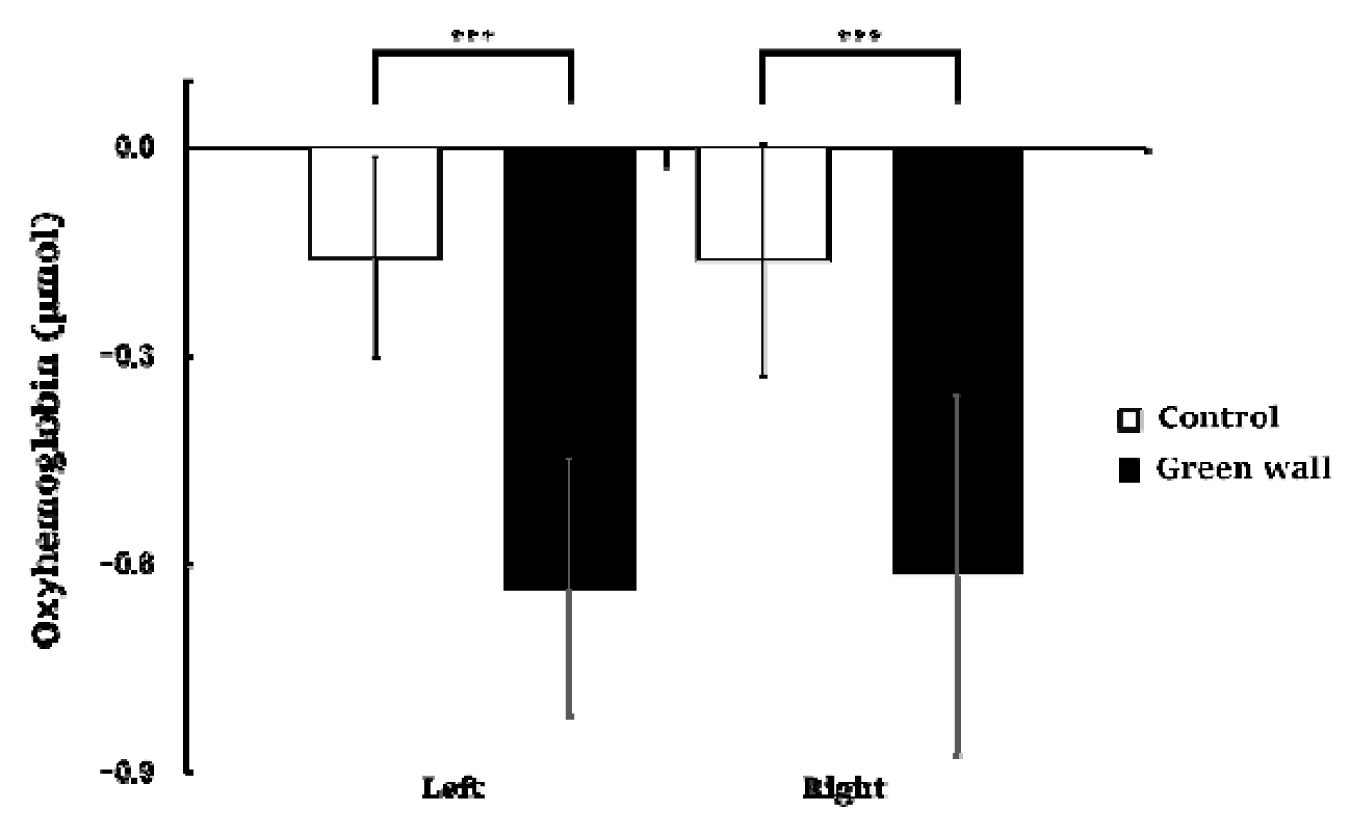
Comparison of oxyhemoglobin concentrations in the control and green walls in the left prefrontal cortex and right prefrontal cortex. N = 11; ***, p < .001; Wilcoxon signed-rank test.
As a result of an analysis dividing the hemodynamic changes of the left and right prefrontal cortex while resting in the green wall room and control room into 60 seconds for each, we discovered that the concentration levels in the left and right prefrontal cortex were generally lower in the green wall room than the control room. For the left prefrontal cortex, the section from 60 to 120 seconds (Control, −0.202 ± 0.111 μmol; Green wall, −0.731 ± 0.122 μmol; p < .001) showed the most significant decrease, showing a more relaxed state than in the hemodynamic change in the control room while maintaining a decrease in concentration (Fig. 5). For oxyhemoglobin concentration in the right prefrontal cortex, there was a decrease in blood flow in the control room in the section from 60 to 120 seconds (Control, −0.154 ± 0.164 μmol; Green wall, −0.537 ± 0.303 μmol; p < .001) and the section from 540 to 600 seconds (Control, −0.236 ± 0.127 μmol; Green wall, −0.548 ± 0.246 μmol; p < .001), but there was an increase in the green wall room. Moreover, in 120 to 180 seconds (Control, −0.251 ± 0.117 μmol; Green wall, −0.727 ± 0.089 μmol; p < .001), 300 to 360 seconds (Control, −0.243 ± 0.110 μmol; Green wall, −0.823 ± 0.350 μmol; p < .001), and 420 to 480 seconds (Control, −0.111 ± 0.207 μmol; Green wall, −0.672 ± 0.082 μmol; p < .001), there was a rapid decline in the green wall room compared to the decline in the control room (Fig. 6).

Comparison of oxyhemoglobin concentrations of control and green walls for the left prefrontal cortex for 60 seconds. N = 11; ***, p < .001; Wilcoxon signed-rank test.
Autonomic changes
As a result of analyzing HRV, BP, and PR to assess changes in cardiovascular function due to rest in the green wall room, we discovered a significant difference in HRV. The parasympathetic nervous system index HF activated when relaxed was higher in the green wall room compared to the control room (Control, 214.8 ± 32.3 msec2; Green wall, 273.9 ± 42.8 msec2; p < .001) (Fig. 7 left), and the sympathetic nervous system index LF/HF tended to be lower in the green wall room compared to the control room (Control, 2 .2 ± 0 .8; Green wall, 1 .5 ± 0 .5; p < .001) (Fig. 7 right). When we analyzed BP and PR, we did not find a significant difference in the control room and green wall room in the systolic BP (Control, 120.1± 12.8 mmHg; Green wall, 120.0 ± 11.1 mm Hg, n.s.), diastolic BP (Control, 73.3 ± 9.5 mmHg; Green wall, 74.4 ± 10.0 mmHg, n.s.), and PR (Control, 7 0.6 ± 8.4 beats/min; Green wall, 7 0.5 ± 6.5 beats/min, n.s.).
Results of psychological measurement
Perceived Restorativeness Scale (PRS)
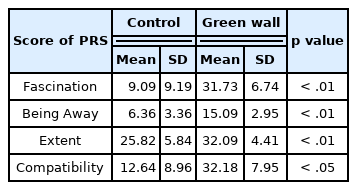
As a result of analyzing the data of the Perceived Restorativeness Scale (PRS) measured on all participants, we discovered that the score was significantly higher in the green wall room compared to the control room (Control, 53.9 ± 21.3; Green wall, 111.1 ± 18.3; p < .01) (Fig. 8) Domains of PRS such as fascination (Control, 9.1 ± 9.2; Green wall, 31.7 ± 6.7; p < .01), being away (Control, 6.4 ± 3.4; Green wall, 15.1 ± 2.9; p < .01), extent (Control, 25.8 ± 5.8; Green wall, 32.1 ± 4.4; p < .01), and compatibility (Control, 12.6 ± 9.0; Green wall, 32.2 ± 7.9; p < .01) also showed similar results (Table 1).
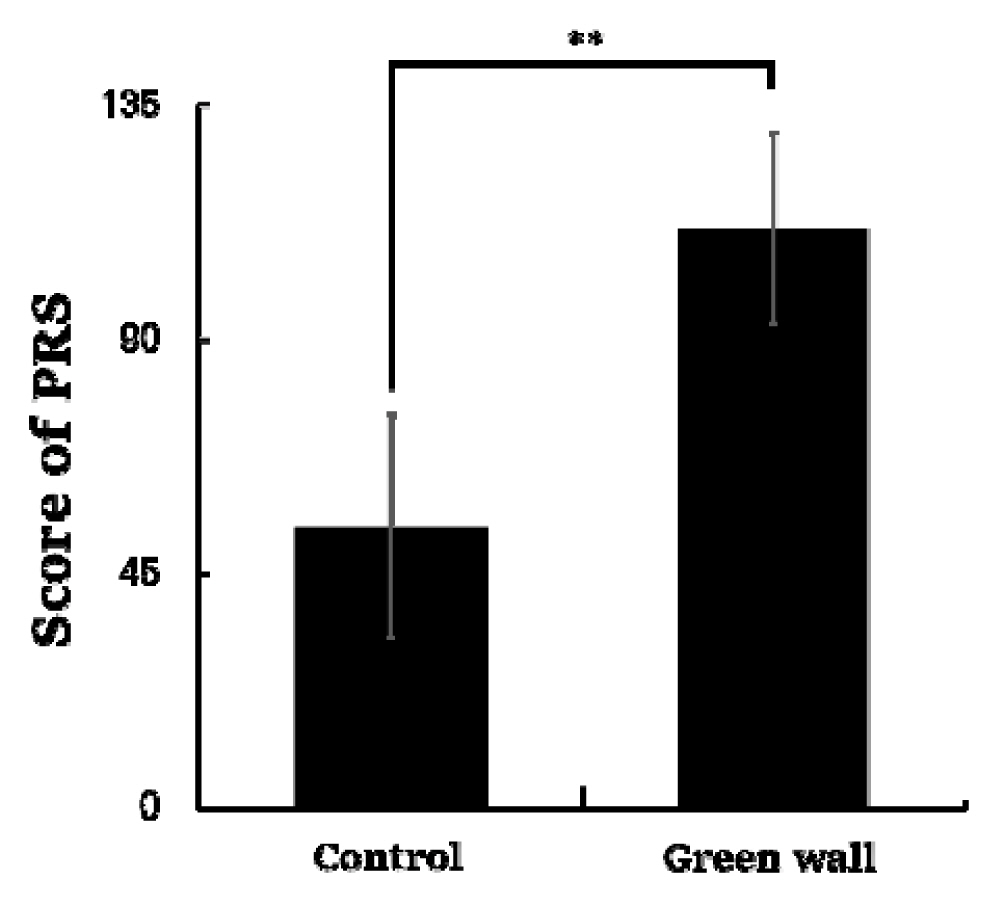
Comparison of PRS total score between control and green wall. N = 11; **, p < .01; Wilcoxon signed-rank test.
Zucherman inventory of personal reaction scale (ZIPERS)
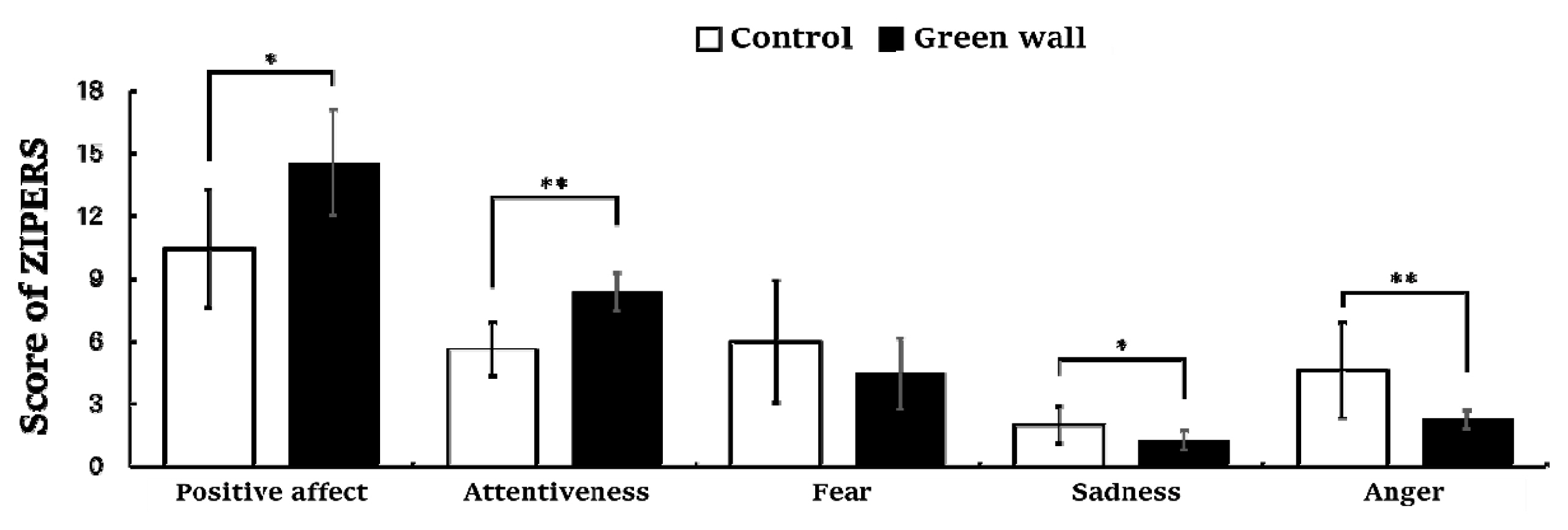
The results of the Zucherman Inventory of Personal Reaction Scale (ZIPERS) measured after resting for 10 minutes in the control room and green wall room showed a significant difference in all five domains except ‘fear.’ Positive affect (Control, 10.5 ± 2.8; Green wall, 14.5 ± 2.5; p < .05) and attentiveness (Control, 5 .6 ± 1 .3; Green wall, 8.4 ± 0.9; p < 0 .01) were higher in the green wall room than in the control room, while sadness (Control, 2.0 ± 0.9; Green wall, 1 .3 ± 0 .5; p < .05) and anger (Control, 4.6 ± 2.3; Green wall, 2.3 ± 0.5; p < . 01) were lower. Moreover, mean value of fear (Control, 6.0 ± 2.9; Green wall, 4.5 ± 1.7; n.s.) was lower in the green wall room than in the control room, but there was no significant difference between them (Fig. 9).
Profile of mood states (POMS)
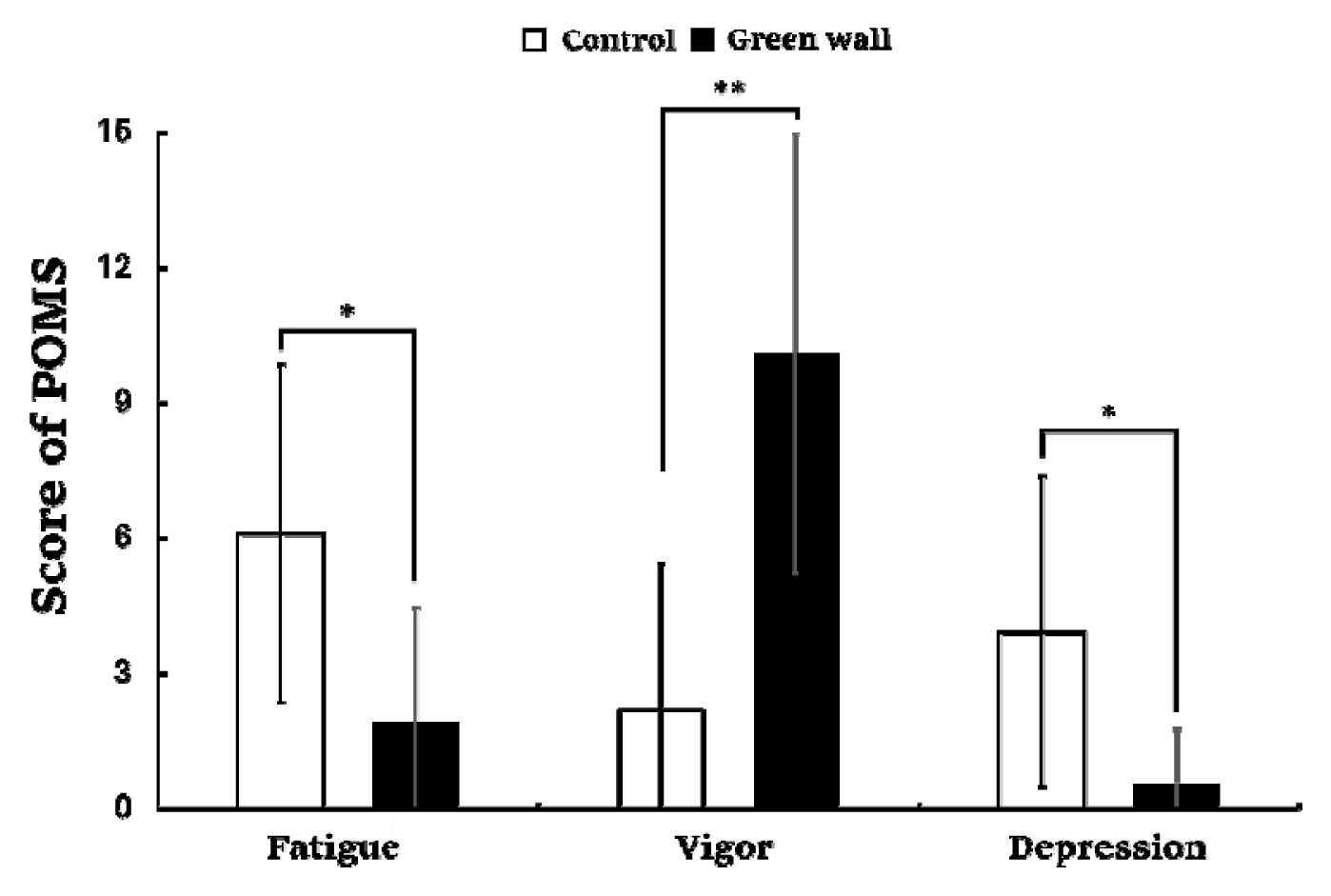
The results of the Profile of Mood States (POMS) used to identify changes in mood states showed a significant difference in each domain. Negative emotions such as fatigue (Control, 6.1 ± 3.8; Green wall, 1.9 ± 2.5; p < .05) and depression (Control, 3.9 ± 3.4; Green wall, 0.5 ± 1.2; p < .05) were lower in the green wall room than in the control room, whereas positive emotions such as vigor (Control, 2.2 ± 3.3; Green wall, 10.1 ± 4.9; p < .01) were higher in the green wall room than in the control room (Fig. 10).
State-trait anxiety inventory (STAI)
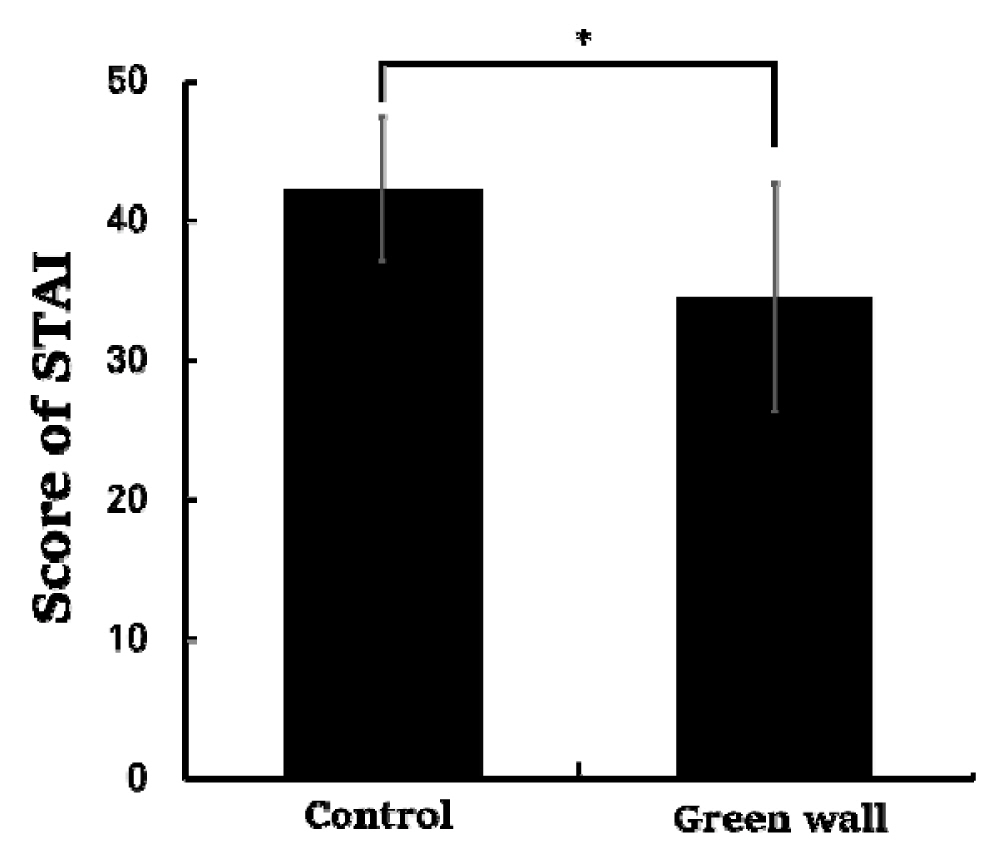
The STAI measuring the anxiety levels of the subjects showed that anxiety levels decreased more significantly while the subjects rested in the green wall room compared to the control room (Control, 42.3 ± 5.2; Green wall, 34.5 ± 8.2; p < .05) (Fig. 11).
Discussion
This study investigated the effects of green walls in medical institutions on the frontal lobe hemodynamic change and autonomic activity, as well as the psychological states of healthcare workers. The results showed that resting in the green wall room resulted in a significantly lower oxyhemoglobin concentration in the prefrontal cortex than resting in the control room. Comparing the oxyhemoglobin concentrations in the left and right prefrontal cortex over 10 minutes, we discovered a similar level of decrease, implying an equal effect on the left and right prefrontal cortex. The decrease in the frontal lobe oxyhemoglobin concentration indicates a decrease in oxygen delivered to the frontal lobe, a concentration change in the resting phase that has been interpreted as a result of physiological relaxation in previous studies (Lee, 2017; Ochiai et al., 2017; Park et al., 2007). Prefrontal cortical activities were closely related to the autonomic response. Participants showed higher level of parasympathetic activity and lower level of sympathetic activity when they were resting in the green wall room. In addition to the physiological effect, there were also high levels of positive emotions like attentiveness and vigor, while negative emotions like fatigue, depression, anger, and anxiety decreased in the green wall room. These results indicate that the green wall might have restoration potential for hospital workers with high levels of stress.
As a result of analyzing the changes in the oxyhemoglobin concentration of the prefrontal cortex, this study showed that prefrontal cortical activity could calm down while resting in the green wall room compared to the control room. The increase and decrease of blood flow in the frontal lobe are proportional to the oxyhemoglobin concentration and inversely proportional to the deoxyhemoglobin concentration (Hoshi et al., 2001), and previous studies interpret a decrease in oxyhemoglobin concentration as a relaxation of brain activity. Previous studies have compared the blood flow of the frontal cortex in students, which changes as they walk and rest in a forest or a city, and proved that activities in a forest reduced blood flow in the left prefrontal cortex more effectively than activities in the city (Park et al., 2007). Other studies have also confirmed that watching 3D natural images using displays reduces oxyhemoglobin concentration in the right prefrontal cortex (Igarashi et al., 2014; Song et al., 2018). Moreover, indoor foliage plants reduced anxiety and depression and lowered the activity of the right prefrontal cortex (Park et al., 2016, 2017). A study on patients with spinal diseases reported that looking at potted plants increased positive emotions like vigor and decreased activity in the left prefrontal cortex (Ochiai et al., 2017). In addition, a study by Lee (2017) that measured the blood flow concentration of the prefrontal cortex in reaction to watching garden images showed a decrease in oxyhemoglobin concentration in both the left and right prefrontal cortex, which is similar to the results of this study. Based on a previous study, the left frontal lobe is related to positive emotions, and the right frontal lobe is related to negative emotions (Canli et al., 1998). The blood flow response of the front lobe using NIRS is a useful tool for investigating the central nervous system’s response to a natural environment. Along with research using fMRI, it provides important evidence for reviewing the correlation between emotional response and cerebral response. Thus, it was proved that indoor green walls applying natural elements could effectively relieve stress and calm down the central nervous system. Although there are many cases in which the hemodynamic changes in the left and right prefrontal cortex are asymmetrical, any generalized conclusions have not yet been drawn on the left and right functions of the frontal lobe. However, there are relatively consistent results on the hemodynamic change in the right prefrontal cortex (Coan and Allen, 2004), which was also found in this study. Further study is required to generalize the characteristics of prefrontal cortex responses to natural environments about the effects considering various factors, such as individual personality or the strength of environmental stimuli.
There was also a positive effect on the autonomic response while resting in the green wall room. According to related studies, walking in a forest promoted parasympathetic activity (Song et al., 2015), and looking at indoor plants increased parasympathetic activity and decreased sympathetic activity (Ikei et al., 2014). Considering that the results of this study are partially consistent with the results of previous studies, it was proved that exposure to various sizes of green walls has a positive effect on autonomic and central nervous activities. Furthermore, previous studies have reported that experiencing green environments can reduce blood pressure and purse rate (Tsunetsugu et al., 2007; Lee et al., 2009; Park et al., 2010), although this study did not show significant differences in these parameters.
In addition to these results, hospital workers showed increased vigor and attentiveness by resting in the green wall room, while negative emotions like fatigue and depression decreased. This study also shows partially consistent results with previous studies stating that indoor plants have a positive effect on the increase of attentiveness and work productivity in workers and are effective in increasing psychological stability and relieving pain for inpatients (Lohr et al., 1996; Park and Mattson, 2008). This implies that elements of the natural environment that comprise a green wall room may contribute to the enhancement of psychological stability and work efficiency of healthcare workers. There was no significant difference in the score of fear, which is not consistent with previous research reporting that natural elements tend to reduce fear (Kim et al., 2022). Additional research is needed on whether the different responses of certain emotions are due to the difference in the experimental conditions or the attributes of the participants. Since this study had relatively small sample size and limited age groups, it is necessary to examine more diverse cases to prove the therapeutic effect of indoor green walls.
Conclusion
This study investigated the evidence that resting in a room with green walls induces relaxation of the brain and cardiac activities for workers in medical facilities and has a positive effect on psychological states. Resting in the green wall room significantly decreased the oxyhemoglobin concentration of the left and right prefrontal cortex while also activating parasympathetic activity and inhibiting the sympathetic activity of the autonomic nervous system. In addition, positive emotions, such as vigor, increased, while negative emotions, such as anxiety, decreased in green wall room. This study indicates that green wall could enhance physiological and psychological stability of hospital workers with high levels of stress. It is suggested that green walls can be an effective tool for reducing stress for individuals who spend many hours in closed indoor space.
Notes
This study was conducted with the support for “A Validation Study on the Effectiveness of Using Smart Gardens” by Korea Arboreta and Gardens Institute