|
 |
- Search
| J. People Plants Environ > Volume 26(1); 2023 > Article |
|
ABSTRACT
Background and objective: This study was conducted to investigate the effect of the cultivation environment of Allium microdictyon in forest cultivation on leaf growth characteristics and antioxidant activity.
Methods: One-year seedlings of Allium microdictyon were planted in test sites with different elevations and slopes in a forest. Each test site was selected on the south-facing slope and north-facing slope each at 700 m and 500 m above sea level, and each test site was created within a straight-line distance of 200m. Leaf characteristics were investigated and antioxidant enzyme activity were measured from April to June the following year after planting.
Results: The 500m sites reached the maximum leaf size about a week earlier than the 700m sites, and the maximum leaf area was doubled in the same periods and higher in the south-facing slope than the north-facing slope. In the south-facing slope in Misan-ri, where the growth was the fastest, the total nitrogen (T-N) in soil also showed a high content. Regarding the antioxidant activity according to the planting site and harvest time, there was little difference in superoxide dismutase (SOD), but catalase increased until the time of release and then decreased thereafter. Malondialdehyde (MDA), a measure of the degree of lipid peroxidation, decreased until the harvest date and then slightly increased.
Conclusion: Based on the results of this study, it is possible to control the harvest date by adjusting the location environment such as the slope direction of the sites for forest cultivation of Allium microdictyon. Catalase and MDA tended to be proportional or inversely proportional depending on the harvest date, serving as most active indicators of change in physiological activity.
Methods: One-year seedlings of Allium microdictyon were planted in test sites with different elevations and slopes in a forest. Each test site was selected on the south-facing slope and north-facing slope each at 700 m and 500 m above sea level, and each test site was created within a straight-line distance of 200m. Leaf characteristics were investigated and antioxidant enzyme activity were measured from April to June the following year after planting.
Results: The 500m sites reached the maximum leaf size about a week earlier than the 700m sites, and the maximum leaf area was doubled in the same periods and higher in the south-facing slope than the north-facing slope. In the south-facing slope in Misan-ri, where the growth was the fastest, the total nitrogen (T-N) in soil also showed a high content. Regarding the antioxidant activity according to the planting site and harvest time, there was little difference in superoxide dismutase (SOD), but catalase increased until the time of release and then decreased thereafter. Malondialdehyde (MDA), a measure of the degree of lipid peroxidation, decreased until the harvest date and then slightly increased.
Conclusion: Based on the results of this study, it is possible to control the harvest date by adjusting the location environment such as the slope direction of the sites for forest cultivation of Allium microdictyon. Catalase and MDA tended to be proportional or inversely proportional depending on the harvest date, serving as most active indicators of change in physiological activity.
Allium microdictyon is a perennial plant that belongs to the Liliaceae family and is a type of wild edible greens that smells of garlic all over (Kim, 2018). In South Korea, it is known to grow naturally in forests of Jirisan Mountain, Seoraksan Mountain, and Ulleungdo Island, or in the northern alpine regions (Lee, 1985). Having been used as a type of wild herbs and vegetables, the importance of Allium microdictyon as a functional food is increasing as its pharmaceutical efficacy has been recently recognized, such as the effect of its extracts on hyperlipidemia and obesity (Choi, 2005), the pharmaceutical efficacy of its extracts for white rats (Choi et al., 2003), and neuroprotective effect (Chung et al., 2015).
Accordingly, studies have been conducted on the cultivation method of wild herbs and vegetables (Kim, 2018; Choi et al., 1998; Ryu et al., 1997; Kim et al., 2010) as well as economic analysis (Park et al., 2014), but most are limited to production through the establishment of cultivation methods such as proper shipping timing and yield improvement. In particular, unlike field cultivation, forest cultivation has a great impact on the generation and growth of understory plants due to light conditions affected by upper-layer stands, and thus research on this is necessary (Choi, 2001).
Growing environments affect the formation of plants including growth, organ differentiation, and primary and secondary metabolites (Shohael et al., 2006), and they also serve as factors of environmental stress in plant growth. Plants must endure stress by using the least amount of energy available through the antioxidant-related mechanism in the body in order to properly resist and defend themselves from stress (Han et al., 2006). In this process, superoxide dismutase (SOD) serves as a catalyst for disproportionation reaction that converts superoxide ion (O2ŌłÆ) to hydrogen peroxide (H2O2) (Bowler et al., 1992), and hydrogen peroxide is converted to water as it is transferred into peroxisomes by catalase (Bowler et al., 1994).
This study was conducted to obtain data on the production quality and relationship of Allium microdictyon leaves depending on the slopes of the cultivation site by investigating the change in antioxidant enzyme activity according to the light conditions of the cultivation site in forest cultivation of Allium microdictyon.
One-year seedlings of Allium microdictyon were planted in April 2015 in test sites with different elevations and slopes in a forest in Inje-gun, Gangwon-do. Each test site was selected on the south-facing slope and north-facing slope each at 700 m and 500 m above sea level, and each test site was created within a straight-line distance of 200m (Table 1). The maximum intensity for each test site was described after measuring the intensity from noon to 2 p.m. during planting. Artificial thinning was not performed in order to cultivate in an environment similar to natural state, and only parts of the herbaceous layer in the understory of the planting site were removed. Leaf length (cm), leaf width (cm), leaf area (cm2), and fresh weight (g) were investigated from April to June the following year after planting. Leaf area was measured using WinFOLIA (Regent Instruments Inc., Canada).
To analyze the chemical properties of the soil with focus on nitrogen (N), phosphoric acid (P), and potassium (K), the organic matter of the O layer was removed from the test sites, after which samples were collected repeatedly three times in the test sites using a soil can. The collected samples were air-dried indoors, pulverized, and passed through a 2 mm sieve. For sample analysis, the total nitrogen content was analyzed by the Kjeldahl method using Foss Kjeltec 2200. The organic content was measured by the Tyurin method, available phosphate content by the Lancaster method, and exchangeable cation content by mixing and shaking 1N-ammonium acetic acid, then filtering and measuring with an atomic absorption spectrophotometer (Analytik Jena AG Nov AA-300, Germany)
0.5 g of the sample was homogenized by adding 10 ml of a mixed solution of 50 mM phosphate buffer (pH 7.0), 10 mM ascorbic acid, and 1.0% (w/v) polyvinylpyrrolidone (PVP), and then centrifuged at 4 ┬░C in 20,000 ├Ś g for 30 minutes, after which the supernatant was used to analyze enzyme activity.
Superoxide dismutase (SOD) activity was measured using the nitroblue tetrazolium (NBT) reduction method, and the absorbance increase was measured at 530 nm for 120 seconds with a spectrophotometer (Beauchamp and Fridovich, 1971), after which it was calculated using the method by Asada et al. (1974).
For catalase activity analysis, 30ul of enzyme extract and 500 ul of 10 mM H2O2 were mixed and reacted at room temperature for 1 minute, after which the reaction was stopped with 500ul sodium acid. 20 ul of the reaction solution and 2 ml of HRP/Chromogen were added again, and absorbance was measured at 520 nm after 10 minutes (Fossati et al., 1980).
Malondialdehyde (MDA) content was measured using the method by Heath and Parker (1968). 10 ml of the mixture containing 6.25 mM of phosphate buffer (pH 7.0) was added to 0.5g of the sample, which was homogenized and then centrifuged at 4 ┬░C in 12,500 rpm (12,000 ├Ś g) for 20 minutes, after which the supernatant was used for analysis. 1 ml of the extract and 1 ml of 0.5% 2-thiobarbituric acid were added, heated for 15 minutes, and rapidly cooled. The absorbance was measured at 532 nm to determine the MDA content.
10 ml of methanol was added to 1g of leaves collected according to the method by Biois (1958) and extracted at room temperature for 24 hours, after which it was centrifuged and 2.5 ml of the supernatant was collected and mixed uniformly with 1ml of 0.3mM DPPH (╬▒,╬▒-diphenyl-╬▓-picrylhydrazyl), and then it was left at room temperature for 30 minutes and measured at 525 nm. The antioxidant effect was expressed as electron donating capacity (%).
The leaf growth of Allium microdictyon reached its maximum between April 13 and April 22, after which there was almost no growth (Table 2). Allium microdictyon is shipped from farms with a length of 20 cm and a width of 10 cm at the least, and the leaf area is 140ŌĆō150 cm2. The point of time when the length and width appeared to be similar to the time of shipment from the farms by test site was April 22 for the south-facing slope in Jeongja-ri, May 22 for the north-facing slope in Jeongja-ri, and April 13 for the south-facing slope in Misan-ri. The south-facing slope showed earlier growth than the north-facing slope. For the north-facing slope in Misan-ri, the root part survived, but shipping was impossible due to the stems damaged by wild animals. Forest cultivation requires more management measures than field cultivation. The reason why the south-facing slope showed relatively faster leaf growth than the north-facing slope is because the south-facing slope was affected by the amount of light (Table 1). Thus, the growth was faster due to physiological factors such as temperature difference between slopes and photosynthesis. Like garlic, an increase in temperature leads to summer decline of Allium microdictyon (RDA, 1999). Thus, the growth stops and the fresh weight decreases as the temperature rises, with the leaves losing vitality, withering, and dropping, thereby showing that fresh weight has decreased over time. There was almost no growth in all test sites after this period. Unlike Ligularia fischeri and Ligularia stenocephala, if the leaves of Allium microdictyon are picked once a year, the leaves to not come out again that same year. Thus, to adjust the harvest time and increase the harvest period, methods such as differentiating the planting sites in consideration of the slope directions can be applied.
Adjusting the cultivation and harvest times of forest products is a key factor of success. Field cultivation is mostly done on bare land, and thus the harvest times are determined mostly by temperature changes. However, in forest cultivation, light conditions can be controlled according to the conditions of the stands, which greatly affects the occurrence and physiological state of understory plants (Choi, 2001; Park and Bae, 2012). High light conditions lead to increased temperature of the cultivation sites. Allium microdictyon is a plant that grows naturally in the alpine area of 1,000m above sea level and in cool areas of Ulleungdo, and the temperature of cultivation is about 8ŌĆō20 ┬░C in July, thereby preventing summer decline in a cool place (RDA, 1999). Therefore, forest cultivation can provide an environment that is closer to native land compared to field cultivation.
Soils by test site showed weak acidity (pH 5.59ŌĆō6.52) regardless of region (Table 3). The acidity was higher than that of general forest soil, which was higher than the pH of the soil where Aster scaber Thunb and Ligularia fischeri are cultivated (pH 5.2) (Choi et al., 2009). The major components shown in the study results of upland soil of cultivation sites for Aster scaber Thunb and Ligularia fischeri are as follows (Choi et al. 2009). Due to phosphoric manure fertilization, the available phosphate in upland soil was 6ŌĆō20 times higher than that in forest land. Calcium, potassium, and magnesium were generally higher in Misan-ri, which showed a fast appearance and growth rate of vegetation. These are all major elements in soil and are directly related to plant growth, thereby showing the result above. Previous studies also revealed that growth such as leaf length and width increased as nitrogen fertilization amount increased when planting Ligularia fischeri (Choi et al. 2009). This study also revealed that total nitrogen content increased in the soil of Misan-ri where leaf growth appeared quickly. It has also been reported that the growth of perilla increases as the amount of phosphatic fertilization increases, and that the aboveground growth is severely inhibited when it is deficient (Choi and Park, 2008). Cation exchange capacity, which activates nutrient absorption by converting anions in soil into cations, was higher than upland soil in all test sites.
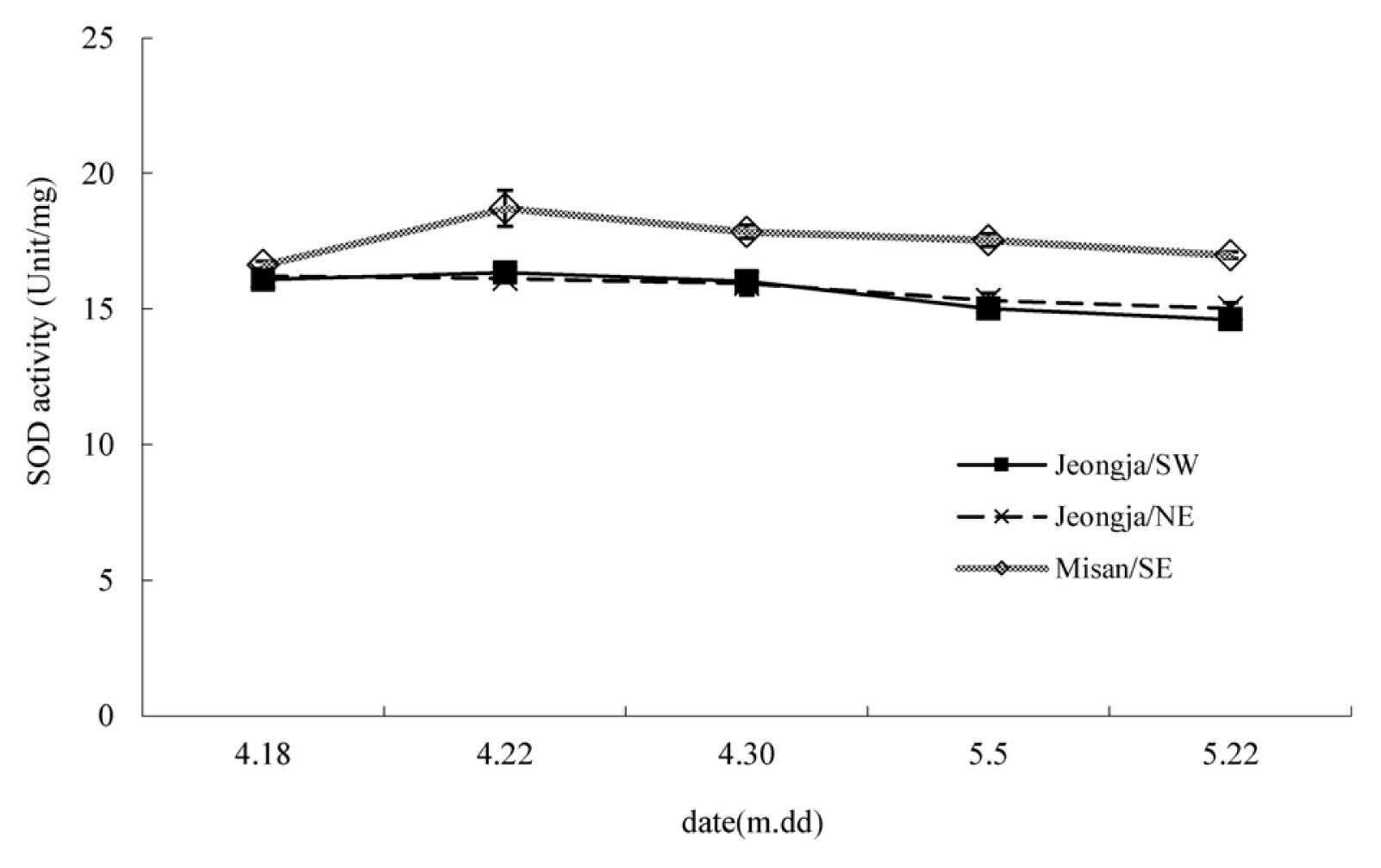
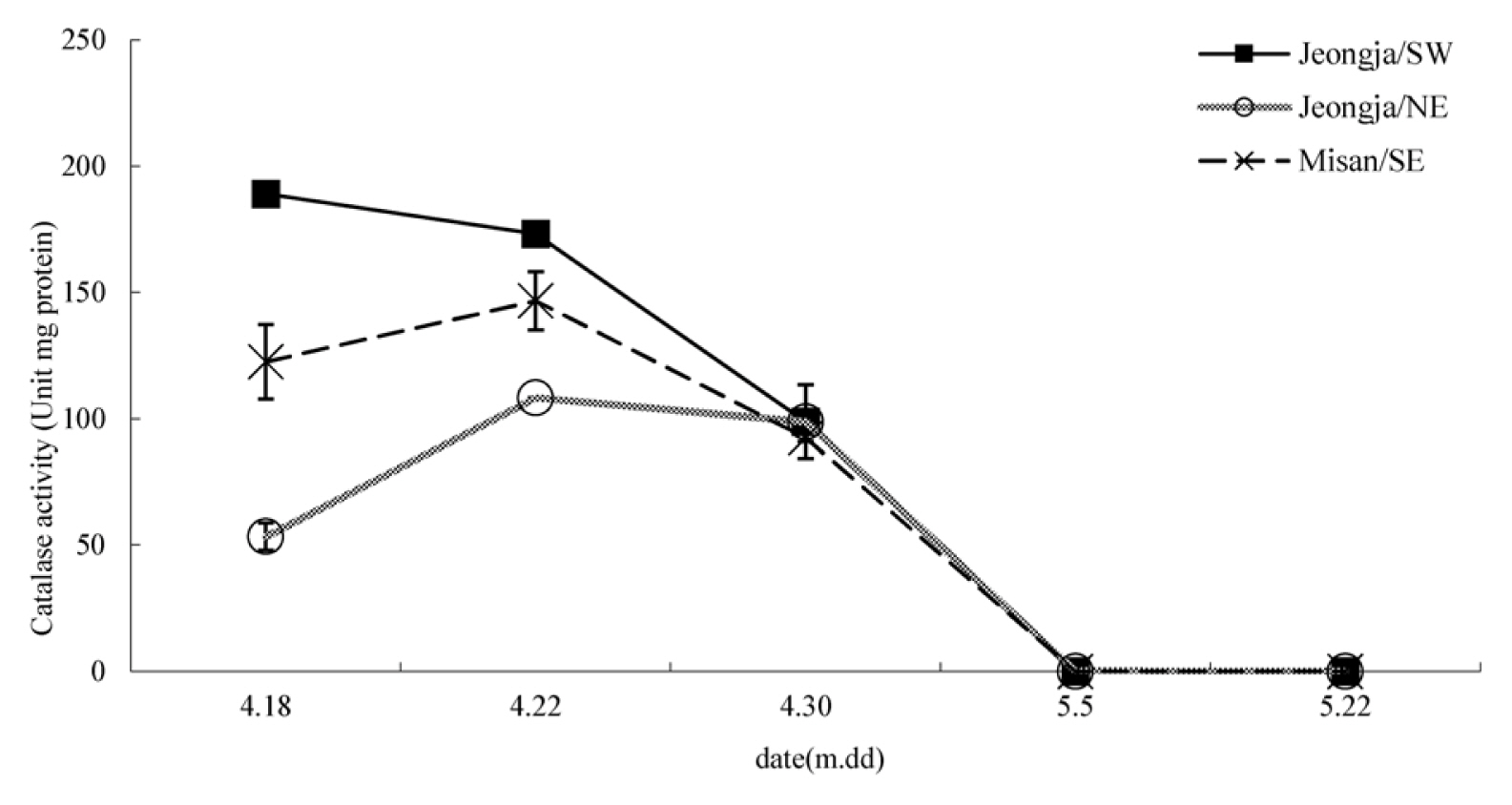
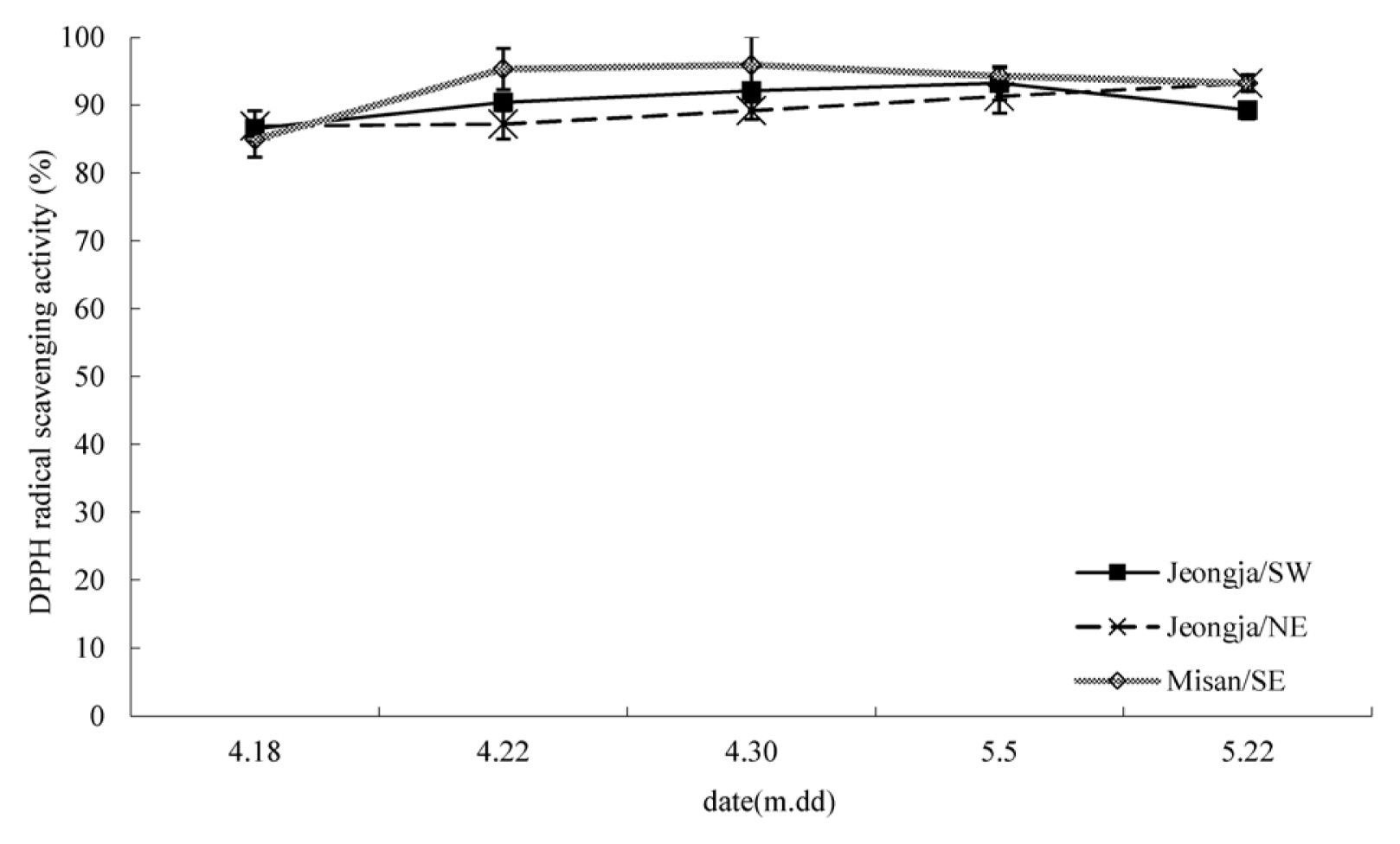
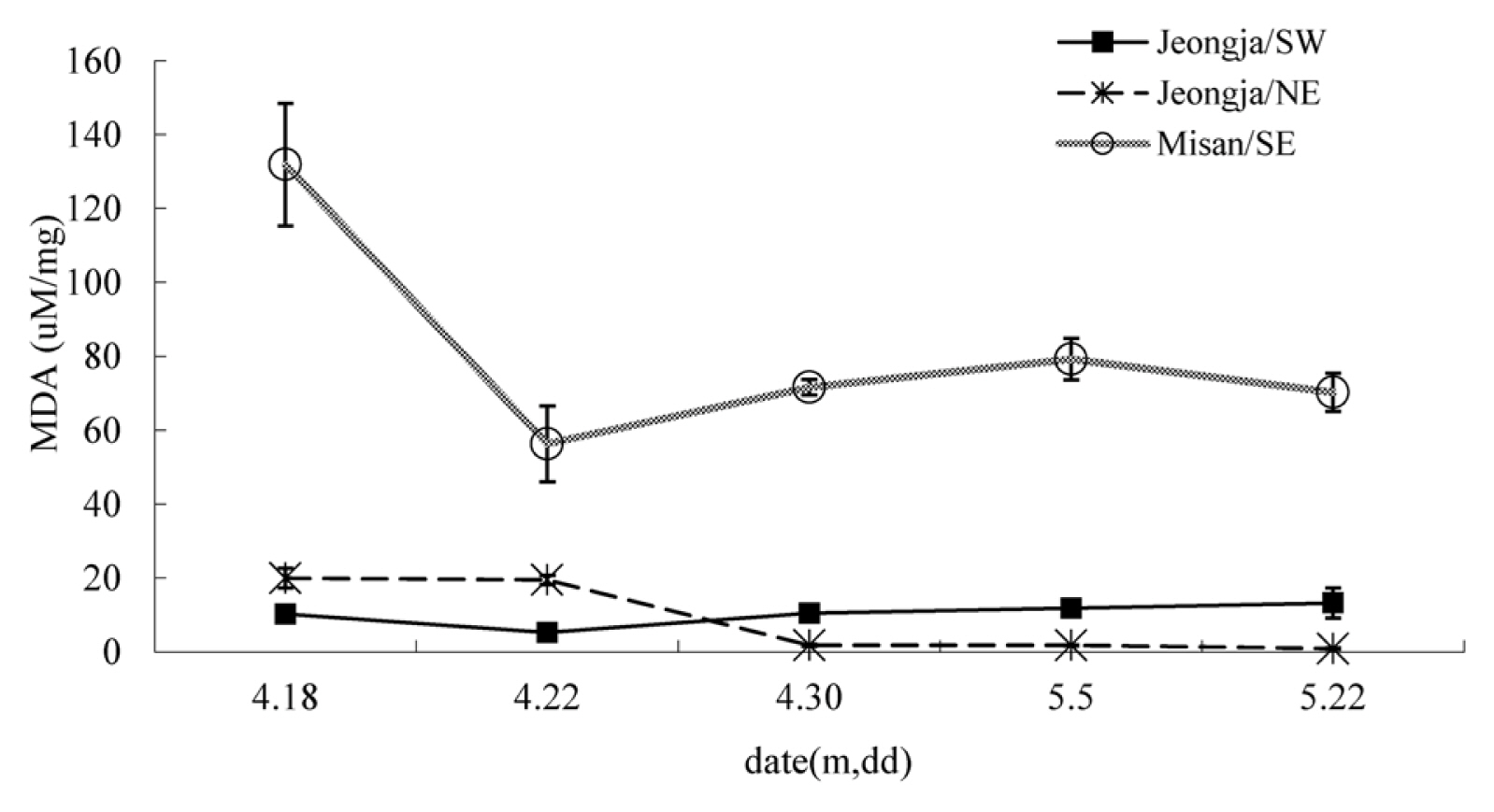
Superoxide dismutase (SOD) showed the highest level overall in the south-facing slope in Misan-ri. The south-facing slope in Jeongja-ri and the north-facing slope in Jeongja-ri generally showed similar values and barely showed any change over time (Fig. 1). The south-facing slope in Misan-ri showed a somewhat high value on April 22, but the difference was not much. Catalase showed a relatively large difference between test sites (Fig. 2). The south-facing slope in Jeongja-ri showed a continuous decline, and the south-facing slope in Misan-ri and north-facing slope in Jeongja-ri showed an increase between April 18 and April 22 and then a decrease after that. Accordingly, the values were similar in all three sites on April 30, after which they all decreased and showed almost no activity by May 5. Plants produce reactive oxygen species under various stress conditions and cope with this stress by producing antioxidant enzymes such as SOD and catalase as a self-defense response (Asada, 1999). SOD plays an important role in the antioxidant system as a catalyst for the disproportionation reaction that converts superoxide ion (O2ŌłÆ) into hydrogen peroxide (H2O2) and oxygen (O2) (Bowler et al., 1992). Hydrogen peroxide is known to reduce damage caused by excessive reactive oxygen species by being decomposed into water molecules and oxygen molecules by peroxidase (POD) or catalase (Anderson et al., 2005; Salin, 1991). Catalase, which showed a large difference in this experiment, continued to decrease on the south-facing slope in Jeongja-ri, which implies that the point at which leaf growth reached its maximum was earlier than in other sites. Both the south-facing slope in Misan-ri and the north-facing slope in Jeongja-ri showed an increase and then a decrease. As the plant grew, its self-defense function also increased, but it decreased at the peak of the growth period, leading to decline and death. Regarding DPPH free radical scavenging activity, Sedum acre showed a huge difference of 61.3%ŌĆō82.4% depending on the regional species when lyophilization was extracted (Kim et al., 2008), but in this study, almost no difference was observed depending on the test site and period (Fig. 3). As a result of measuring the malondialdehyde (MDA) content to find out the degree of lipid peroxidation, the south-facing slope in Misan-ri showed a big change, showing a significant decrease from April 18 to April 22 when there was a great change in growth, thereby reaching its minimum (Fig. 4). The south-facing slope in Jeongja-ri also showed a minimum value on April 22, but the change was smaller than that of Misan-ri. On May 5 when the harvest time was the latest, MDA content was almost nonexistent compared to other regions. Plants show different activities depending on the growth period. Alnus firma leaves that grow naturally in abandoned mine areas showed lowest activity in April and highest activity in June (Han et al., 2006). Ligularia fischeri showed more effective harvest in May compared to early harvest (April) (Suh et al., 2020). It is believed that there is a difference in the change of the active period depending on plant species.
Based on the results of this study, it is possible to control the harvest date up to 30 days by adjusting the location environment such as the slope direction of the sites for forest cultivation of Allium microdictyon. Catalase and MDA tended to be proportional or inversely proportional depending on the harvest date, serving as most active indicators of change in physiological activity during harvest.
Table┬Ā1
General condition of forest cultivation sites
Table┬Ā2
The leaf biomass as affected by cultivated sites
| Location | Slope | Leaf | date(m/dd) | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 4/13 | 4/22 | 4/30 | 5/5 | 5/22 | |||
| Jeongja-ri | SW | Length(cm) | 16.8*┬▒0.08 | 20.50┬▒0.58 | 21.33┬▒0.33 | 21.67┬▒0.24 | 21.94┬▒0.11 |
| Width(cm) | 9.17┬▒0.25 | 12.13┬▒0.17 | 13.25┬▒0.28 | 13.79┬▒0.21 | 14.45┬▒0.23 | ||
| Area(cm2) | 80.45┬▒5.48 | 141.57┬▒7.54 | 146.64┬▒7.65 | 149.13┬▒5.15 | 148.99┬▒4.01 | ||
| Fresh weight(g) | 8.04┬▒0.46 | 7.79┬▒0.34 | 5.36┬▒0.50 | 4.16┬▒0.16 | 3.94┬▒0.49 | ||
|
|
|||||||
| Jeongja-ri | NE | Length(cm) | 9.92┬▒0.38 | 15.61┬▒1.38 | 15.42┬▒1.47 | 17.03┬▒1.85 | 19.01┬▒0.48 |
| Width(cm) | 6.00┬▒0.33 | 8.90┬▒1.47 | 8.64┬▒1.12 | 9.08┬▒1.23 | 10.53┬▒0.91 | ||
| Area(cm2) | 37.48┬▒3.50 | 80.54┬▒4.64 | 87.06┬▒4.54 | 98.37┬▒5.78 | 125.45┬▒5.97 | ||
| Fresh weight(g) | 2.64┬▒0.24 | 2.52┬▒0.28 | 3.61┬▒0.32 | 3.43┬▒0.31 | 2.94┬▒0.49 | ||
|
|
|||||||
| Misan-ri | SE | Length(cm) | 20.85┬▒0.21 | 21.31┬▒0.42 | 21.30┬▒0.47 | 21.90┬▒0.16 | 22.86┬▒0.46 |
| Width(cm) | 12.12┬▒0.06 | 8.90┬▒1.47 | 8.64┬▒1.12 | 9.08┬▒1.23 | 10.53┬▒0.91 | ||
| Area(cm2) | 140.01┬▒8.21 | 145.99┬▒4.21 | 146.41┬▒3.47 | 146.40┬▒2.14 | 147.01┬▒3.46 | ||
| Fresh weight(g) | 7.94┬▒0.54 | 7.45┬▒0.46 | 5.96┬▒0.45 | 4.20┬▒0.16 | 3.81┬▒0.16 | ||
|
|
|||||||
| Misan-ri | NE | Length(cm) | - | - | - | - | - |
| Width(cm) | - | - | - | - | - | ||
| Area(cm2) | - | - | - | - | - | ||
| Fresh weight(g) | - | - | - | - | - | ||
Table┬Ā3
Soil contents of cultivated area
| Location | pH (1:5*) | EC (dS/m*) | OM (g/kg) | P2O5 (mg/kg) | Ca | K | Mg | CEC | T-N (%) |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| (cmol(+)/kg ) | |||||||||
| Jeongja/ SW | 5.68 | 0.17 | 58.0 | 25 | 3.53 | 0.18 | 0.56 | 12.70 | 0.22 |
| Jeongja/ NE | 5.60 | 0.09 | 42.3 | 9 | 2.89 | 0.08 | 0.56 | 10.28 | 0.29 |
| Misan/ SE | 5.84 | 0.24 | 72.3 | 17 | 11.56 | 0.41 | 1.92 | 18.67 | 0.42 |
| Misan/ NE | 6.52 | 0.34 | 81.9 | 31 | 15.14 | 0.78 | 2.06 | 24.08 | 0.53 |
References
Anderson, M.D., T.K. PrasadStewart, C.R. Stewart. 1995. Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiology. 109(4):1247-1257. https://doi.org/10.1104/pp.109.4.1247



Asada, K.M., Takahashi, M, Nagate. 1974. Assay and inhibitors of spinach superoxide dismutase. Agricultural and Biological Chemistry. 38:471-473. https://doi.org/10.1080/00021369.1974.10861178

Asada, K. 1999. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology. 50:601-639. https://doi.org/10.1146/annurev.arplant.50.1.601


Beauchamp, C., I. Fridovichi. 1971. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry. 44(1):276-287. https://doi.org/10.1016/0003-2697(71)90370-8


Blois, M.S. 1958. Antioxidant determination by the use of a stable free radical. Nature. 181:1199-1200. https://doi.org/10.1038/1811199a0

Bowler, C., M. Van Montagu, D. Inz├®. 1992. Superoxide dismutase and stress tolerance. Annual Review of Plant Physiology and Plant Molecular Biology. 43:83-116. https://doi.org/10.1146/annurev.pp.43.060192.000503

Bowler, C., W. Van Camp, M. Van Montagu, D. Inz├®. 1994. Superoxide dismutase in plants. Critical Reviews in Plant Sciences. 13:199-218. https://doi.org/10.1080/07352689409701914

Bradford, M.M. 1976. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72(1ŌĆō2):248-254. https://doi.org/10.1016/0003-2697(76)90527-3


Choi, B.G., S.B. Bang, S.B. Kwon. 1998. Research on the development of regionally specialized crops: A study on the comparison of characteristics of Allium microdictyon seedlings and the establishment of cultivation methods. Gangwondo Agric. Res. Ext. Ser. 734-736. (in Korean). Retrieved from https://lib.rda.go.kr/search/searchDetailART.do?ctrl=000001078860&marcType=zf&lang=kor&loca=rda00001&sysDiv=TOT&siteCode=home&sMenu=3010¶mStrlink=siteCode%3dhome%26amp%3bamp%3bsType%3dKWRD%26amp%3bamp%3bq%3d%25EC%2582%25B0%25EB%25A7%2588%25EB%258A%2598%26amp%3bamp%3bsysDiv%3dTOT%26amp%3bamp%3bnDt%3d%26amp%3bamp%3bsi%3dTOTAL%26amp%3bamp%3bloca%3drda00001%26amp%3bamp%3bsMenu%3dnull
Choi, J.H. 2001. Effects of artificial shade treatment on the growth performances, water relations, and photosynthesis of several tree species. Doctoral dissertation Chungnam National University. Daejeon, Korea:
Choi, J.W., K.T. Lee, W.B. Kim, K.G. Park, H.J. Jung, H.J. Park. 2003. Pharmcological effects of the Allium victorialis varplatyphyllum extracts on the rats induced by Streptozotocin, Poloxamer-407, CCl4 and D-Galactosamine. Korean Journal of Pharmacognosy. 34(3):250-255.
Choi, J.W., K.T. Lee, W.B. Kim, K.K. Park, W.Y. Chung, J.H. Lee, S.C. Lim, H.J. Jung, H.J. Park. 2005. Effect of Allium victorialis varplatyphyllum leaves on triton WR-1339-induced and poloxamer-407-induced hyperlipidemic rats and on diet-induced obesity rats. Korean Journal of Pharmacognosy. 36(2):109-115.
Choi, S.C., S.Y. Ahn, M.S. Ahn, Y.S. Ok, J.S. Son, J.H. Joo. 2009. Effect of nitrogen application rate on growth and yields of Aster scaber Thunband Ligularia fischeri Turcz. in the first year after transplanting. Korean Journal of Environmental Agriculture. 28(3):243-248. https://doi.org/10.5338/KJEA.2009.28.3.243

Choi, J.M., J.W. Park. 2008. Growth, deficiency symptom and tissue nutrient contents of leaf perilla (Perilla frutesens Britt) influenced by phosphorus concentrations in fertigation solution. Horticultural Science & Technology. 26(1):21-28.
Chung, M.J., Y.I. Park, K.H. Kwon. 2015. Neuroprotective effects of Cirsium setidens, Pleurospermum kamtschaticumin, and Allium victorials based on antioxidant and p38 phoshorylation inhibitory activities in SK-N-SH neuronal cells. Journal of the Korean Society of Food Science and Nutrition. 44(3):347-355. https://doi.org/10.3746/jkfn.2015.44.3.347

Fossati, P., L. Prencipe, G. Berti. 1980. Use of 3,5-dichloro-2-hydroxy benzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clinical chemistry. 26(2):227-231.


Han, S.H., J.C. Lee, C.Y. Oh, J.K. Kim, P.K. Kim. 2006. Seasonal changes of pigment content and antioxidant capacity in leaves of Alnus firma at polluted area. Korean Journal of Agricultural and Forest Meteorology. 8(2):115-116.
Heath, R.L., L.L. Parker. 1968. Photoperoxidation in isolated chloroplasts. I. Kinetics and stiochiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics. 125(1):189-198. https://doi.org/10.1016/0003-9861(68)90654-1


Kim, H.J., S.H. Jung, J.H. Bae, S.Y. Lee. 2008. Growth chracteristics, Vitamin C content and antioxidative activity among local strains of Sedum sarmentosum. Journal of Bio-Environment Control. 17:110-115.
Kim, G.N., M.S. Cho, K.W. Kwon. 2010. Analysis growth performance and ascorbic acid contents of Allium victorialis varplatyphyllum, Ligularia fischeri, and L. stenocephala under changing light intensity. Journal of Korean Society of Forest Science. 99(1):68-74.
Kim, K.K. 2018. Cultivation of edible mountain herbs Jeonju, Jeollabuk-do: Rural Development Administration.
Lee, C.B. 1985. Korean Illustrated Plant Book Seoul: Hyangmoonsa.
Ryu, S.Y. 1997. Establishment of highland Allium microdictyon cultivation technology system: Proper harvesting period and low-temperature treatment period for breaking the dormancy of Allium microdictyon bulbs. Rural Development Administration; 213.(in Korean) Retrieved from https://lib.rda.go.kr/search/searchDetailART.do?ctrl=000000296348&marcType=zh&lang=kor&loca=rda00001&sysDiv=TOT&siteCode=home&sMenu=3010¶mStrlink=isRefine%3dY%26amp%3bamp%3bsiteCode%3dhome%26amp%3bamp%3btitle%3d%26amp%3bamp%3bauthor%3d%26amp%3bamp%3bpublisher%3d%26amp%3bamp%3bpublisher_year%3d%26amp%3bamp%3bprint_acsson_no%3d%26amp%3bamp%3blocation%3d%26amp%3bamp%3bctrl%3d%26amp%3bamp%3bmarcType%3d%26amp%3bamp%3bsMenu%3d%26amp%3bamp%3breturnPrevUrl%3d%26amp%3bamp%3bnDt%3d%26amp%3bamp%3bsysDiv%3dTOT%26amp%3bamp%3bsType%3dKWRD%26amp%3bamp%3blang%3dkor%26amp%3bamp%3bsi%3dTOTAL%26amp%3bamp%3bsi%3dTOTAL%26amp%3bamp%3bq%3d%25EC%2582%25B0%25EB%25A7%2588%25EB%258A%2598%26amp%3bamp%3bq%3d%25EC%259D%25B8%25EA%25B2%25BD%26amp%3bamp%3bb%3dand%26amp%3bamp%3bb%3dand%26amp%3bamp%3brf%3d%26amp%3bamp%3brt%3d%26amp%3bamp%3bmsc%3d500%26amp%3bamp%3bloca%3drda00001%26amp%3bamp%3brefine%3dY
Park, B.M., J.H. Bae. 2012. Effect of shading levels on the growth and chlorophyll contents of Allium victorialis L. var. platyphyllum Makino. Journal of Bio-Environment Control. 21(3):281-285.
Park, S.B., M.J. Kim, E.G. Kim. 2014. Comparison of profitability for Allium victorialis farming system between on-field and under-forest. Journal of Korean Society of Forest Science. 103:122-128. https://doi.org/10.14578/jkfs.2014.103.1.122

Salin, M.L. 1991. Chloroplast and mitochondrial mechanism for protection against oxygen toxicity. Free Radical Research Communications. 12ŌĆō13:851-858. https://doi.org/10.3109/10715769109145867Cite

Shohael, A.M., M.B. Ali, K.W. Yu, E.J. Hahn, R. Islam, K.Y. Paek. 2006. Effect of light on oxidative stress, secondary metabolites and induction of antioxidant enzymes in Eleutherococcus senticosus somati embryos in bioreactor. Process Biochemistry. 41(5):1179-1185. https://doi.org/10.1016/j.procbio.2005.12.015

Suh, J.T., K.D. Kim, H.B. Sohn, S.J. Kim, S.Y. Hong, Y.H. Kim. 2020. Comparative study of antioxidant activities at different cultivaiton area and harvest date of the Gomchwi ŌĆśSammanyŌĆÖ variety. Korean Journal of Plant Resources. 33:245-254. https://doi.org/10.7732/kjpr.2020.33.4.245

- TOOLS
-
METRICS

-
- 1 Crossref
- 520 View
- 16 Download
- Related articles in J. People Plants Environ.
-
Landscape Characteristics of
Sojinjeong Garden in Geochang2022 December;25(6)Soil Characteristics of Specialized Plant Garden in Korea National Arboretum2017 February;20(1)